Infectious Disease: Most Read Stories From 2024
Drug Topics
DECEMBER 28, 2024
Check out this list of our top 5 most read infectious disease stories from the year 2024.
Drug Topics
DECEMBER 28, 2024
Check out this list of our top 5 most read infectious disease stories from the year 2024.
Pharmacy Times
DECEMBER 28, 2024
Study results show that the updated BNT162b2 XBB vaccine is effective in preventing hospitalizations or emergency department visits associated with COVID-19 complications.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
DECEMBER 30, 2024
Although bipartisan legislation to provide Medicare coverage for obesity treatments has circulated in Congress for more than a decade, the Congressional Budget Office (CBO) ventured to estimate the budgetary effects of such a policy for older Americans starting in 2026 for the very first time in a report published in October. The CBO should be commended for conducting this comprehensive analysis.

pharmaphorum
DECEMBER 30, 2024
BMS beats MSD to the US subcutaneous PD-1 inhibitor market after Opdivo Qvantiq is cleared across nearly all the indications of the IV form

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Topics
DECEMBER 29, 2024
Check out this list of our top 5 most read womens health stories from the year 2024.
Pharmacy Times
DECEMBER 30, 2024
Anthony Perissinotti, PharmD, BCOP, discusses unmet needs and trends in managing chronic lymphocytic leukemia.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

The Checkup by Singlecare
DECEMBER 30, 2024
Sold under brand names such as Amvaz, Katerzia, Norliqva, and Norvasc , amlodipine is a medication that is FDA approved for the treatment of hypertension (high blood pressure), chronic stable angina (chest pain and pressure), vasospastic angina (coronary artery spasm), and coronary artery disease. It belongs to a class of medications called calcium channel blockers, which is further broken down into two classes: dihydropyridine calcium channel blockers and nondihydropyridine calcium channel bloc

Pharmaceutical Commerce
DECEMBER 30, 2024
A dive into the top articles in this sector making waves this year.
Pharmacy Times
DECEMBER 30, 2024
Plaintiff argues that "blacklisting certain individuals with opioid prescriptions was unlawful.
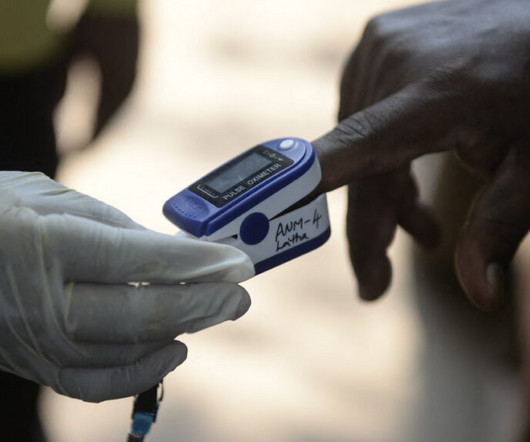
STAT
DECEMBER 30, 2024
Work by device manufacturers to improve the performance of pulse oximeters on people with darker skin has progressed little since the Food and Drug Administration asked manufacturers in 2013 to voluntarily test the devices on more diverse skin tones, according to a study published Monday in JAMA. The study and a related editorial suggest clearer guidance, enforcement, and possibly legal action may be necessary to ensure the devices work well on all skin tones.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharmaceutical Technology
DECEMBER 30, 2024
The US FDA has granted approval for Bristol Myers Squibbs (BMS) Opdivo Qvantig for subcutaneous use to treat various solid tumours.

PharmExec
DECEMBER 30, 2024
In this part of his Pharmaceutical Executive video interview, Dr. Lawrence B. Werlin, MD, FACOG of HRC Fertility (@md.lawrence.werlin on TikTok), how new radiological techniques are revolutionizing the diagnosis of conditions like endometriosis and the impact it will have on patients.

Pharmacy Times
DECEMBER 30, 2024
The disparity highlights opportunities for pharmacists to get involved and address health disparities.

European Pharmaceutical Review
DECEMBER 30, 2024
The use of advanced therapies using cells or genes to achieve significant leaps in medical treatment was perceived as a niche sector just a decade ago, but it has now started to garner a greater level of understanding and recognition in the pharmaceutical industry. Numerous groundbreaking advances have shown these therapies hold potential to revolutionise how we treat, and potentially cure, many conditions, including for patients with underlying genetic or cellular causes of disease that have

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmaceutical Technology
DECEMBER 30, 2024
Tevimbra is already approved in the US for the treatment of unresectable or metastatic oesophageal squamous cell carcinoma (ESCC).

PharmExec
DECEMBER 30, 2024
OncLive serves as the connection to oncology, including groundbreaking cancer news and interviews with top oncologists in multimedia formats.
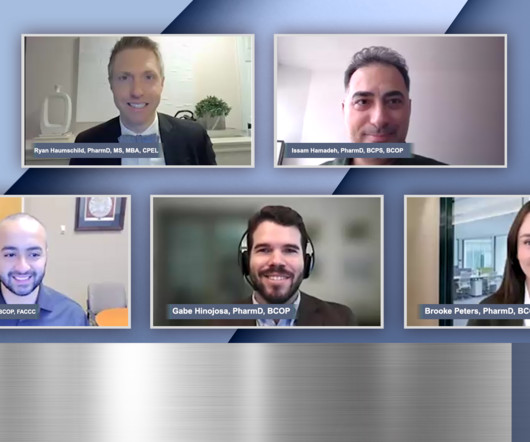
Pharmacy Times
DECEMBER 30, 2024
Panelists discuss understanding the comparative advantages, decision-making factors, infrastructure requirements, and partnership models for administering bispecific antibodies in community vs academic settings, with particular focus on patient care logistics and referral pathways.
Hospital Pharmacy Europe
DECEMBER 30, 2024
A study looking at the use of potentially inappropriate medications (PIMs) in hospitalised adults aged75 and over has highlighted the need for multidisciplinary interventions to review chronic medications in these patients. The study found that the overall prevalence of PIMs was moderate but varied considerably between hospitals and also revealed a potential missed opportunity to optimise medication use within this setting.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

pharmaphorum
DECEMBER 30, 2024
Learn how digital biomarkers are revolutionising respiratory care by providing personalised, real-time data for better monitoring and management of respiratory diseases.

Med Ed 101
DECEMBER 29, 2024
Reducing diabetes medications in geriatric patients with low A1c involves careful consideration of the risks and benefits of continued intensive glycemic control. In older adults, particularly those with multiple comorbidities, frailty, or limited life expectancy, maintaining very low A1c levels may increase the risk of adverse outcomes, such as hypoglycemia, which can lead to falls, […] The post Deprescribing in Diabetes – Geriatric Focus appeared first on Med Ed 101.
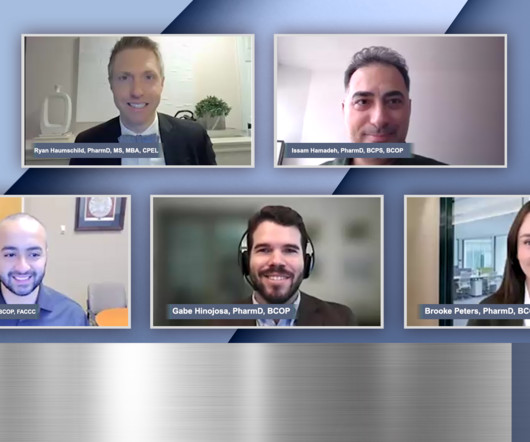
Pharmacy Times
DECEMBER 30, 2024
Panelists discuss how key recommendations for optimizing bispecific therapy care focus on establishing robust communication protocols between academic and community centers while ensuring community centers develop comprehensive infrastructure including staff training, emergency protocols, and care coordination pathways.

Pharmaceutical Technology
DECEMBER 29, 2024
Nelistotug is under clinical development by GSK and currently in Phase II for Recurrent Head And Neck Squamous Cell Carcinoma.

pharmaphorum
DECEMBER 30, 2024
Daiichi Sankyo has claimed its first approval, in Japan, for TROP2-targeting antibody-drug conjugate (ADC) datopotamab deruxtecan

STAT
DECEMBER 29, 2024
Former President Jimmy Carter’s oft-stated desire was to see the last Guinea worm die before he did. Though America’s 39 th president, who died Sunday at age 100, did not quite achieve that dream, he left a huge legacy in the field of global health. The causes he espoused are diseases whose names most of us barely know. Onchocerciasis, or river blindness.

Pharmacy Times
DECEMBER 28, 2024
Pharmacists are crucial in educating patients, identifying eligible individuals, and promoting uptake of the newly expanded RSV vaccination recommendations for older adults.

Pharmaceutical Technology
DECEMBER 29, 2024
Trastuzumab rezetecan is under clinical development by Jiangsu Hengrui Medicine and currently in Phase I for Head And Neck Cancer.

Pharmaceutical Technology
DECEMBER 29, 2024
Trastuzumab rezetecan is under clinical development by Jiangsu Hengrui Medicine and currently in Phase I for Transitional Cell Carcinoma (Urothelial Cell Carcinoma).

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase II for Hodgkin Lymphoma (B-Cell Hodgkin Lymphoma).

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase II for Diffuse Large B-Cell Lymphoma.

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase I for Lupus Nephritis.

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase II for Follicular Lymphoma.

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase II for Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma).

Pharmaceutical Technology
DECEMBER 29, 2024
GCC-4001 is under clinical development by Artiva Biotherapeutics and currently in Phase II for Angioimmunoblastic T-Cell Lymphoma (AITL)/Immunoblastic Lymphadenopathy.
Let's personalize your content