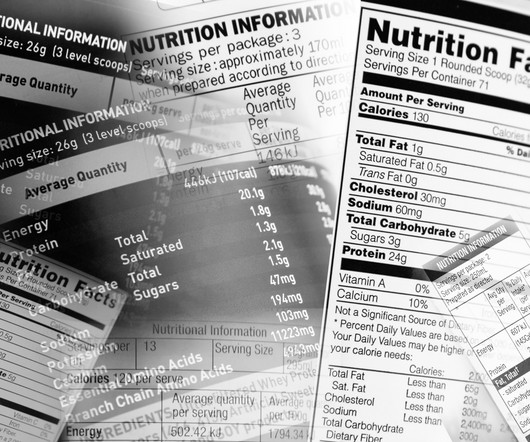
FDA Proposes Front-of-Package Nutrition Label for Most Packaged Foods
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.

European Pharmaceutical Review
MAY 28, 2024
A report by The Brainy Insights has predicted that the ampoules packaging market will value $9.83 This is expected to be largely driven by increasing demand for this packaging type. Considering material type, glass ampoule packaging was found to hold a market share of around 77 percent in 2023, in comparison to plastic.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
SEPTEMBER 12, 2023
After years of deliberation, the FDA has proposed giving patients a simple one-pager for every prescription drug with the information needed for safe and effective use. It wants patients to know them, too.

European Pharmaceutical Review
AUGUST 30, 2023
The agreement stipulates that certain conditions must be met in the labelling and packaging of these medicinal products. After this date: Under the framework, medicines can have the same packaging and labelling across the UK. UK packaging must carry a clearly legible ‘UK only’ label to be allowed onto the UK market.

European Pharmaceutical Review
SEPTEMBER 29, 2023
A paper published in the IEEE Sensors Journal has demonstrated 100 percent inline pharmaceutical packaging content verification by combining standard diffuse reflectance MOEMS-EC-QCL spectroscopy with artificial intelligence (AI). Current quality control challenges in pharmaceutical packaging Flores et al. Flores et al.
European Pharmaceutical Review
OCTOBER 5, 2022
For most consumers, product packaging represents their first impression of a brand and its sustainability credentials. But how is this push shaping the pharma packaging market and what are the key changes we can expect to see? For many, aesthetic appeal and user experience top the list of priorities when it comes to packaging design.

DiversifyRx
APRIL 6, 2023
If you provide strip or multi-dose packaging, you were just handed a golden egg from CVS. CVS suddenly announced the discontinuation of its strip packaging services. This decision has left many patients needing to find a new pharmacy that offers similar packaging services. You can read their announcement HERE.

STAT
DECEMBER 1, 2022
At a time when many Americans are clamoring for more transparency into prescription drug pricing, one key provider of that data is making it harder to access the information. This includes details such as separate doses and packaging on both current and historical prices.

pharmaphorum
JANUARY 9, 2025
Pharma groups have called for EU adoption of electronic product information (ePI) to take place within four years in a new position paper.

European Pharmaceutical Review
JANUARY 9, 2025
Three industry bodies have produced a joint position paper highlighting recommendations for adopting a harmonised implementation of electronic Product Information (ePI) leaflets. It will also enable more patient-centric and accessible content by providing the most up-to-date information.

The FDA Law Blog
JUNE 26, 2024
Lenz, Principal Medical Device Regulation Expert — FDA recently released a new eSTAR template for device pre-submissions and 513(g) Requests for Information, referred to as PreSTAR. A 513(g) Request for Information is a means of obtaining FDA’s views about the classification and regulatory requirements for a particular device.

STAT
JUNE 4, 2024
But a year ago, Zynex informed her that the Tufts plan had never paid, and instead, those bubble-wrapped packages were going to cost her almost $1,000. Before she bought the electrical stimulation unit, Zynex assured her the supplies would be covered by Tufts Health Plan, her insurance company.

STAT
JULY 23, 2024
The committee found evidence of PBMs sharing patient information among affiliated companies for the specific and anticompetitive purpose of steering patients to pharmacies a PBM owns. Continue to STAT+ to read the full story…

The FDA Law Blog
FEBRUARY 2, 2025
The rules were implemented to enhance patient safety protections via revised drug handling, packaging, and delivery requirements. The rules include changes to the notification process, medication packaging, the handling of reports, and safety issues. Hyman, Phelps & McNamara, P.Cs

European Pharmaceutical Review
JANUARY 10, 2023
In 2024, Pharmapack Europe, the two-day exhibition and conference for pharma’s drug delivery and packaging industry will introduce two brand new zones to the flagship event. Bio packaging zone . Pharmapack’s bio packaging zone builds on its long history of being at the forefront of innovations in biological drug delivery.

IDStewardship
NOVEMBER 29, 2023
BCPS, BCIDP Article Posted 1 December 2023 Podcasting has emerged as an incredible way to reach a global audience and discuss scientific information. They are very valuable because they provide information in a way that is readily accessible. Interviewee: Luis Plaza Interviewer: Timothy P. Gauthier, Pharm.D.,

Roots Analysis
AUGUST 14, 2024
Packaging is the process of enclosing a product in a container in order to protect and store it. In the pharmaceutical industry, packaging plays a crucial role in ensuring the strength and integrity of a product. Further, the type of raw material utilized for packaging highly depends on the type of product / drug to be enclosed.

STAT
APRIL 26, 2024
The goal is for the House to pass the bill before the July 4 recess, setting it up to be included in a year-end must-pass legislative package. national security if they give the Chinese government access to sensitive health information about Americans.

ISPE
OCTOBER 1, 2022
Module Type Package: Turning Visionary Concepts into Reality. Module Type Package: Turning Visionary Concepts into Reality. The Module Type Package (MTP) approach designates each module with a digital description, enabling flexible module connection and orchestration. Trudy Patterson. Sat, 10/01/2022 - 06:00. Sponsored Content.

Pharmaceutical Technology
JUNE 14, 2023
Navigating the complexities of pharma and biotech packaging services can be difficult. With the growing number of complex therapies that require specialized packaging and handling requirements, selecting the right contract packaging organization (CPO) involves evaluating what services and additional benefits they can bring to your business.

ISPE
MARCH 9, 2023
The following CoP’s will be represented: Advanced Therapy Medicinal Products (ATMPs) Biotechnology Commissioning & Qualification Combination Products Containment Critical Utilities Disposables/Single-Use Technologies GAMP ® Investigational Products Oral Solid Dosage Pharma 4.0
Hospital Pharmacy Europe
MARCH 31, 2025
Their insights into the supply chain allow them to identify inefficiencies and advocate for more sustainable practices, such as reducing excess packaging and improving storage conditions. J Am Med Inform Assoc 2024;31(3):73245. Self-reported digital literacy of the pharmacy workforce in North East Scotland. 8 Alowais M et al.
The Checkup by Singlecare
JANUARY 9, 2025
Youll know your semaglutide isnt compounded if you spot the brand name on the packaging and information leaflet. Its also packaged differently, which can be an easy identifier. Brand-name semaglutide comes in two formspre-filled injectable pens (Ozempic and Wegovy) and oral tablets (Rybelsus).
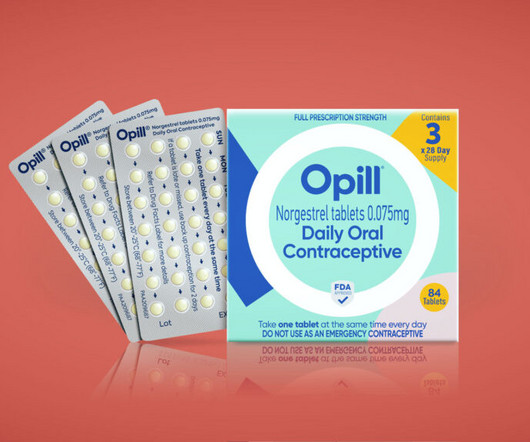
STAT
JULY 27, 2023
It’s not every day that a marketing team is tasked with designing the branding and packaging of the first over-the-counter birth control pill to be sold in the U.S.

Roots Analysis
FEBRUARY 1, 2024
Further, the cold chain storage process involves the cold chain storage, cold chain transportation, and monitoring temperature controlled packaging products. Therefore, the pharmaceutical, biotech, food and chemical industries usually rely on cold chain packaging for the transportation of their products.
European Pharmaceutical Review
NOVEMBER 24, 2022
One area in which the problem can be tackled effectively is product packaging. With predictions that the global market for anticounterfeiting packaging is set to reach almost $250 billion by 2026, growth in authentication and anti-tamper devices such as holograms appear to have a healthy future.”

STAT
JULY 11, 2024
This calls for celebration with a cup of stimulation, and we are opening a new package of blueberry muffin for the occasion. The agency sent the drugmaker a complete response letter, saying it needs more information on the drug’s manufacturing process, as well as more data on how well it performs in people with type 1 diabetes.

European Pharmaceutical Review
AUGUST 31, 2023
To cope with the growing demand for production flexibility and agility, IMA Group is structured according to highly specialised pharmaceutical divisions that concentrate on specific processing and packaging areas. Isolation technologies and other containment solutions are also part of our expertise.

European Pharmaceutical Review
AUGUST 23, 2023
It offers information for manufacturers of new and established biopharmaceuticals on ways to incorporate them into their quality testing. A separate informational chapter was developed to expand on the use, validation, and comparability of endotoxin tests based on recombinantly derived substances.

ISPE
MARCH 28, 2023
Thanks to the clinical supply chain service companies' years of industry development and operation, there are new developments in global supply chain layout, ultra-low temperature storage and distribution management breakthroughs, and growing packaging and labeling capabilities.

European Pharmaceutical Review
DECEMBER 6, 2023
Elaborating on supply chain disruptions impacting sterile injectables, Venkatesh explains how the supply of e.g. packaging material and filters has not yet caught up demand following the pandemic. Packaging solutions for track and trace are becoming increasingly important and can be considered emerging trends.

European Pharmaceutical Review
JANUARY 25, 2024
Therefore, extractables and leachables assessment should consider the packaging components of the CCS, including the labelling. However, according to the FDA, this is “less of a concern” for products, such as biological products, that are packaged in glass containers.

pharmaphorum
JANUARY 12, 2022
The cobas pulse system is a point-of-care device that combines a glucose test strip reader with a touch screen that is used for displaying patient information and data, as well as analysing and sharing clinical results.

European Pharmaceutical Review
JUNE 4, 2024
IMA Safe Specialised in primary and secondary packaging, IMA Safe creates blister-packaging machines, capsule and tablet counters, sachet and stick-packaging machines, tube fillers and cartoners. End-of-line solutions, from robotics, handling, overwrapping to case-packing and palletising are provided by the IMA EOL hub.

pharmaphorum
NOVEMBER 24, 2020
The elements of its digital health portfolio included integrated care management unit Thrasys – whose SyntraNet health information exchange platform is used to organise patient health records and workflows.

European Pharmaceutical Review
JANUARY 23, 2023
Through EMA’s engagement with its stakeholders, the Medicines Shortages Steering Group has received up-to-date information from community pharmacists on the situation in pharmacies across the EU. Based on current information from companies and stakeholders, it is expected that the situation will improve in the coming months.
European Pharmaceutical Review
JULY 25, 2022
These may be mislabelled or produced in fake packaging and, most dangerously, there is no regulation around their manufacture. As a result, the development of advanced packaging-level tokens has led to watermarking techniques; invisible, encoded data that requires specialist verification software. Supply-chain visibility.

European Pharmaceutical Review
JANUARY 25, 2024
Within the EU, patient information leaflets (PILs) are not merely a regulatory requirement but a cornerstone of patient safety. These standardised documents provide meticulously curated and scientifically approved information. Exploring the benefits of ePI ePI offers advantages, notably swift access to updated medication information.

European Pharmaceutical Review
JULY 27, 2023
With this innovation, researchers can package biological materials into tablets that can be stored on a shelf at room temperature. How the method was tested The research team demonstrated that the cell’s machinery capable of decoding genetic information into making RNA and proteins can be stored in a solid-state.

STAT
JULY 2, 2024
This calls for celebration with a cup of stimulation, and we are opening a new package of maple bourbon for the occasion. The agency sent a civil investigative demand requiring Teva to provide information related to the patents listed in the so-called Orange Book, which is maintained by the U.S. Spring has sprung, after all.

European Pharmaceutical Review
MARCH 21, 2024
Insights obtained from patients are used to better understand the impact of the disease on their daily life, and to carefully design all stages of drug product manufacture: from clinical studies to package design. What are the three main factors to consider when looking to develop patient-centric drug products?

STAT
AUGUST 21, 2023
At a meeting at Cold Spring Harbor Laboratory that drew more than 400 of the field’s top scientists, the company shared more detailed information about studies it has conducted to assess the risks that renegade CRISPR components could be getting into an egg or sperm.
European Pharmaceutical Review
AUGUST 23, 2022
On- or in-dose authentication means that a security measure or anticounterfeit feature is integrated with the dosage form itself, offering product verification and traceability embedded into each medicine, rather than on the secondary package” WHO estimates that >50 percent of the drugs for sale on the internet are fake.

The FDA Law Blog
AUGUST 27, 2023
the ability to associate the saleable return product with the transaction information/statement with the particular product). the ability to associate the saleable return product with the transaction information/statement with the particular product). Drug manufacturers have had electronic systems in place since 2017. Guidance at 4.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content