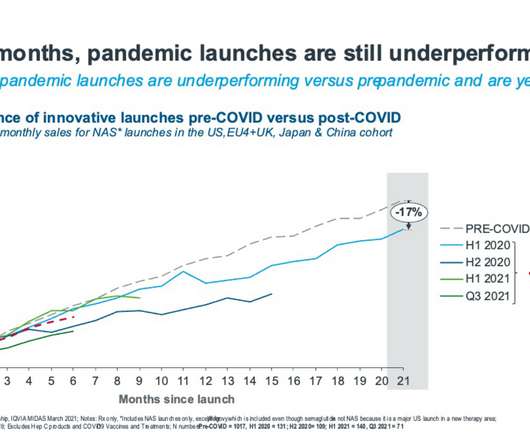
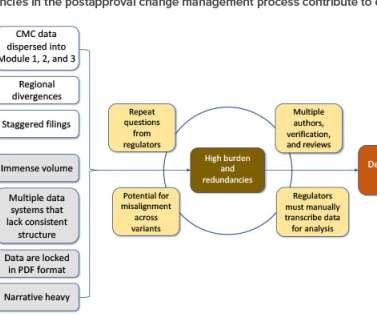
Race to the finish: pharma edges closer to approval with RSV vaccines
Pharmaceutical Technology
FEBRUARY 1, 2023
RSV researchers at major pharmaceutical companies are currently working to develop new RSV drugs to beat future waves of RSV infection and gain the first RSV vaccine FDA approval. Pharmaceutical companies are pushing to develop drugs and vaccines for RSV with these populations in mind.

















Let's personalize your content