HDA 2023 Traceability Seminar: An FDA DSCSA Overview
Pharmaceutical Commerce
AUGUST 30, 2023
Event’s first session aims to analyze the legislation’s implementation, as FDA enforcement is delayed until November 2024.

Pharmaceutical Commerce
AUGUST 30, 2023
Event’s first session aims to analyze the legislation’s implementation, as FDA enforcement is delayed until November 2024.

Pharmaceutical Commerce
AUGUST 27, 2024
The agency provides an update surrounding the Act’s implementation efforts.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Commerce
AUGUST 28, 2024
In an interview with Pharma Commerce Editor Nicholas Saraceno, Jeb Hunter, Senior Regulatory Consultant, EAS Consulting Group, discusses the on “What to Expect When They’re Inspecting: FDA Inspections on DSCSA Compliance" breakout session at the 2024 HDA Traceability Seminar.

The FDA Law Blog
JANUARY 11, 2024
This day-long (FREE) seminar is a must-attend event for anyone in the pharmaceutical sector interested in understanding the “ins and outs” of doing business in the Territory, as well as an opportunity to interact with those who already have a keen understanding of doing business in Puerto Rico.

pharmaphorum
SEPTEMBER 28, 2020
All this comes at a time when COVID-19 has made attending medical conferences and seminars in person impractical because of social distancing regulations. Mohty said: “I think the Medscape FDA results are a wonderful example of how you can positively impact a given field, and I think we should really move in this direction.”.
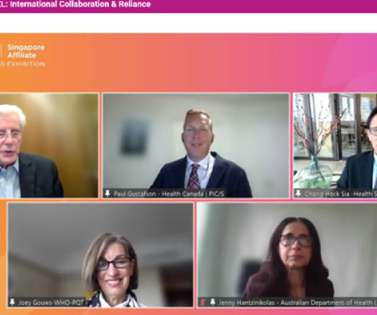
ISPE
OCTOBER 28, 2022
It was usual practice for TGA to prepare additional guidance/explanatory documents for industry and for TGA inspection staff to participate in training seminars to assist industry. The outcomes of these inspections were shared extensively with MRA partners such as EMA and US FDA. Canada DHPID ). Canada DHPID ).
ISPE
NOVEMBER 21, 2022
It was usual practice for TGA to prepare additional guidance/explanatory documents for industry and for TGA inspection staff to participate in training seminars to assist industry. The outcomes of these inspections were shared extensively with MRA partners such as EMA and US FDA. The approach by TGA and HSA were very similar.
Let's personalize your content