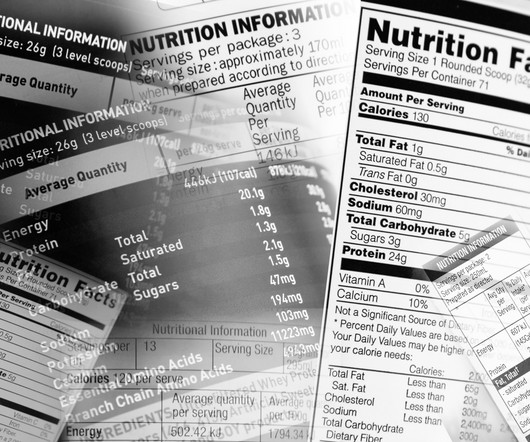
FDA Proposes Front-of-Package Nutrition Label for Most Packaged Foods
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.

STAT
JUNE 20, 2023
WASHINGTON — The FDA wants to make it easier for consumers to know if the foods they’re buying are unhealthy — but doing so is harder than it seems here in the United States. Now, the Food and Drug Administration is embarking on a major study to test front of package labels here in the U.S.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
SEPTEMBER 26, 2024
— Food additives rarely face FDA scrutiny once they’re on the market, even those with mounting evidence of safety concerns. The public meeting on the FDA’s campus drew dozens of food safety advocates, industry representatives, concerned citizens, and state and federal lawmakers. SILVER SPRING, Md.

STAT
DECEMBER 17, 2024
The language of the government funding package has not been publicly released as of Monday night and could still change, but sources indicate some longtime health care policy priorities are making the cut for the year-end spending deal, Rachel Cohrs Zhang reports.
The Checkup by Singlecare
JANUARY 9, 2025
Food and Drug Administration (FDA). Why and when semaglutide is compounded According to the FDA, compounded drugs are made by licensed pharmacists (or under the supervision of pharmacists in outsourcing facilities) who combine, mix, or alter ingredients of a drug. There are a few times when compounding is necessary.

STAT
SEPTEMBER 12, 2023
After years of deliberation, the FDA has proposed giving patients a simple one-pager for every prescription drug with the information needed for safe and effective use. The Food and Drug Administration knows many of the answers. It wants patients to know them, too.

Pharmafile
APRIL 14, 2023
Pharma giant Eli Lilly’s Biologic Licence Application (BLA) for its bowel disease drug has been declined by the FDA over concerns about the proposed manufacturing of the drug. However, concerns weren’t expressed over the clinical data package, safety or label for the medicine.

Pharmacy Times
AUGUST 28, 2023
The start date of the package-level electronic tracking system will shift from November 27, 2023 to November 27, 2024.

Fierce Pharma
FEBRUARY 5, 2024
If a new package of pivotal data satisfies the FDA, GSK could have another run at the U.S. | If a new package of pivotal data satisfies the FDA, GSK could have another run at the U.S. multiple myeloma market with its previously withdrawn BMCA-targeted antibody-drug conjugate, Blenrep.

European Pharmaceutical Review
OCTOBER 17, 2023
The US Food and Drug Administration (FDA) has published draft guidance on how it intends to use alternative tools for assessing drug manufacturing facilities identified in pending marketing applications. Fundamentally, this approach will establish whether facilities meet applicable requirements for FDA approval and licensure decisions.

Roots Analysis
APRIL 20, 2022
On an average, around 50 drugs are approved by the US Food and Drug Administration (US FDA) annually. This continuously growing pipeline of pharmaceutical drug products has inadvertently led to an increase in the demand for their associated primary packaging and Secondary Packaging solutions. . trillion in 2023.

European Pharmaceutical Review
AUGUST 23, 2022
A study of the causes of warning letters issued by the US Food and Drug Administration (FDA)’s Center for Drug Evaluation and Research (CDER) and Center for Devices and Radiological Health (CDRH) between 2010 and 2020 revealed that poor current good manufacturing practice (cGMP) compliance and misbranding were the most common citations.

Lifewell Rx Pharmacy
JUNE 9, 2021
For example, packaging plays a vital role in promoting medication adherence. A retail pharmacy may use blister packs , Dispill®, and other types of special packages to organize prescriptions and label medications according to dosage and time of intake. Effective packaging stretches a drug’s purpose? b>Medication Information.

The Checkup by Singlecare
JULY 14, 2023
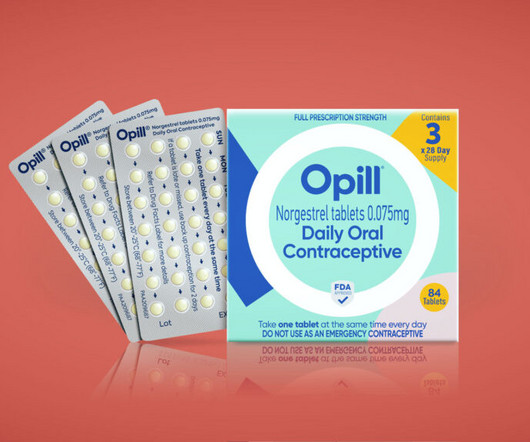
Food and Drug Administration (FDA) approved the first over-the-counter birth control pill , Opill (norgestrel), on July 13. According to the FDA, there are 3 million unintended pregnancies in the U.S. Today’s FDA decision is a historic moment for health equity, sexual health, and reproductive rights. … How does Opill work?

European Pharmaceutical Review
MAY 2, 2023
The US Food and Drug Administration (FDA) has released a new draft guidance to further support the use of decentralised clinical trials (DCTs) for drugs, biologics and devices. The FDA’s regulatory requirements for investigations of medical products are the same for DCTs and traditional site-based clinical trials.

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. Ophthalmic drug products should be evaluated for extractables and leachables, FDA asserted.

The Checkup by Singlecare
MARCH 31, 2023
Food and Drug Administration (FDA) approved a non-prescription version of Narcan (naloxone), a nasal spray used to rapidly reverse the effects of an opioid overdose. According to the FDA, drug overdose is a persistent public health problem in the United States. This week the U.S. chief medical officer of Toolbox Genomics.

STAT
OCTOBER 7, 2022
The FDA has announced the set of rules it proposes to enforce for manufacturers to claim that a food product is “healthy.” ” The proposed rules are a lot better than the labeling anarchy that currently exists. But here’s my bottom line: health claims are not about health. They are about selling food products.

STAT
OCTOBER 31, 2024
Of course, this calls for a cup of stimulation, and we are opening a new package of pecan pie for the occasion. With all doses now listed as available, the FDA may soon pull it off the list, depending on its conversations with Novo about whether the company can sufficiently meet demand going forward. Sound familiar?

European Pharmaceutical Review
NOVEMBER 14, 2023
In response to supply chain challenges, including shortages of critical glass vials for products like COVID-19 vaccines and parenteral drugs, the Packaging and Distribution Expert Committee (PD EC) has made a significant revision of United States Pharmacopeia (USP) General Chapter <660>—Glass.

Roots Analysis
AUGUST 26, 2024
In today’s global landscape, sustainability has emerged as a pivotal concern across various sectors including the pharmaceutical packaging industry. What is Sustainable Packaging / Green Packaging? The following figure presents the raw materials used by the sustainable packaging companies.

STAT
OCTOBER 19, 2022
After years of deliberation, the FDA recently announced a new set of rules it proposes to regulate claims on food packaging that a product is “healthy.” ” The most basic rule: the product must actually contain food, not just ingredients.

The FDA Law Blog
JULY 10, 2023
After a firm submits a 510(k) to FDA, FDA will request still more information after a first-pass review. According to the 2 nd Quarter FY2023 MDUFA V Performance Report , FDA issued a request for additional information (AI request) on the first FDA review cycle for 63% to 68% of 510(k)s submitted in FY2018 to FY2022.

pharmaphorum
AUGUST 6, 2020
The FDA has approved GlaxoSmithKline’s multiple myeloma drug Blenrep, a first-in-class potential blockbuster that will be used in advanced disease. The post FDA approves GSK’s Blenrep for advanced multiple myeloma appeared first on. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the cohort.
STAT
JULY 27, 2023
It’s not every day that a marketing team is tasked with designing the branding and packaging of the first over-the-counter birth control pill to be sold in the U.S.

Pharmaceutical Technology
AUGUST 5, 2024
Reinforced with additional data, Zevra’s arimoclomol data package has gained a positive opinion in Niemann-Pick disease type C from an FDA advisory committee.

The FDA Law Blog
FEBRUARY 2, 2025
The rules were implemented to enhance patient safety protections via revised drug handling, packaging, and delivery requirements. The rules include changes to the notification process, medication packaging, the handling of reports, and safety issues.

STAT
OCTOBER 27, 2022
This calls for celebration with a cup of stimulation, and we are opening a new package of Pumpkin Spice for the occasion. However, this is also shaping up as a beautiful day as well, given the clear and sunny skies — and delicious breezes — enveloping the Pharmalot campus this morning. Autumn, after all, is in full swing.

The FDA Law Blog
FEBRUARY 21, 2024
Mullen — More than five years after FDA first announced its plan to harmonize 21 CFR Part 820 with ISO 13485, on February 2, 2024, FDA finally issued the Quality Management System Regulation (QMSR) Final Rule. FDA further retained some definitions in the QSMR. Notably, Part 820 will look different. Revised § 820.3

The FDA Law Blog
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

STAT
MAY 17, 2023
Simply put, this occurs when a drug company combines two or more medicines in a package deal for health plans and pharmacy benefit managers, which determine lists of medicines that are covered by insurance. The FDA is slated to make a decision on whether to clear the shot in August. Continue to STAT+ to read the full story…
Pharmaceutical Technology
JULY 26, 2022
The US Food and Drug Administration (FDA) has accepted the biologics licence application (BLA) of Novartis subsidiary Sandoz for natalizumab, a proposed first-ever multiple sclerosis (MS) biosimilar to reference medicine Tysabri. A complete analytical, preclinical and clinical data package is part of the BLA and MAA submissions.

pharmaphorum
NOVEMBER 16, 2022
has announced that its platinum-resistant ovarian cancer drug ELAHERE (mirvetuximab soravtansine-gynx) has been granted FDA accelerated approval. RxDx Assay – Roche’s immunohistochemistry (IHC) companion diagnostic for identifying ovarian patients eligible for ELAHERE – has been contemporaneously approved by the FDA. ImmunoGen, Inc.

The People's Pharmacy
MAY 8, 2023
Why, then, are FDA inspections abroad so infrequent and disappointing? FDA Inspections Suspended: During the COVID pandemic, the Food and Drug Administration suspended its inspections at most foreign pharmaceutical facilities. Even under the best of conditions, the FDA never inspects all foreign pharmaceutical plants annually.

The FDA Law Blog
AUGUST 27, 2023
However, FDA recognizes that some “technical and operational issues,” including those involving downstream trading partners and other “affected stakeholders,” may not be fully resolved by the deadline. So what is FDA going to do? By Karla L. Drug manufacturers have had electronic systems in place since 2017. Guidance at 4.

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. In addition to providing additional examples of tree nuts, the draft guidance states that FDA considers the following categories of fish to be major food allergens under Section 201(qq): Jawless fish (e.g.,

STAT
SEPTEMBER 19, 2023
About last night… House leadership last night canceled a scheduled vote on a health care package that included transparency requirements for hospitals and insurers, reforms to some PBM practices, and a small site-neutral payment policy for administering drugs, Rachel writes.
The Checkup by Singlecare
DECEMBER 17, 2024
Like semaglutide, Mounjaro (tirzepatide) is a once-a-week injectable medication that is FDA approved for helping manage blood sugar levels in those with Type 2 diabetes. It mimics two gastrointestinal hormones: glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP).

The FDA Law Blog
SEPTEMBER 27, 2023
The Washington Nationals won the World Series, Presidential administrations have come and gone, and FDA has added new meeting types and formats to its menu. And so, FDA has issued a new draft guidance to bring everyone up to speed on formal meetings under PDUFA. Tobolowsky — Much has changed since the long-gone days of 2017.

pharmaphorum
FEBRUARY 23, 2022
The FDA has issued a complete response letter to Mallinckrodt for terlipressin, on the grounds that the company had been forced to change its packaging and labelling manufacturing facility for the drug. The post FDA knocks back Mallinckrodt’s kidney drug terlipressin appeared first on.

pharmaphorum
SEPTEMBER 15, 2022
Mallinckrodt has finally claimed FDA approval for terlipressin as a treatment for hepatorenal syndrome (HRS), after manufacturing problems scuppered an earlier attempt. The drug is already approved for this indication in dozens of other countries around the world, including much of Europe, Australia and New Zealand.

The Checkup by Singlecare
MARCH 10, 2025
The medication is available in both brand-name and compounded forms, although the compounded forms are not FDA approved. Some insurance plans may cover Ozempic for managing Type 2 diabetes , which is an indication that is approved by the Food and Drug Administration (FDA) to treat. Does insurance cover Ozempic? affected by a shortage).

Pharmaceutical Technology
APRIL 24, 2023
The US Food and Drug Administration (FDA) has granted orphan drug designation (ODD) to XORTX Therapeutics’ oxypurinol to treat autosomal dominant polycystic kidney disease (ADPKD) patients. The company noted that the ODD from the FDA is not an approval for the use of XORLO, a formulation of oxypurinol.

STAT
JULY 11, 2024
This calls for celebration with a cup of stimulation, and we are opening a new package of blueberry muffin for the occasion. This comes after the FDA in May convened a group of advisers to discuss the drug, called icodec. What is upon us right now, however, is our ever-growing to-do list. Sound familiar? Have a great day, everyone.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content