FDA Requires Abrysvo, Arexvy RSV Vaccine Labels to Include Guillain-Barré Warning
Drug Topics
JANUARY 8, 2025
Patients who receive either Pfizer or GSKs respiratory syncytial virus vaccine may be at increased risk of Guillain-Barr syndrome.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Drug Topics
JANUARY 8, 2025
Patients who receive either Pfizer or GSKs respiratory syncytial virus vaccine may be at increased risk of Guillain-Barr syndrome.

Pharmaceutical Technology
MAY 15, 2024
The US agency rejected the expanded use of Dynavax’s vaccine for adults on haemodialysis, citing insufficient efficacy and safety data.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
OCTOBER 25, 2023
Five months after becoming the first company to secure FDA approval for a respiratory syncytial virus (RSV) vaccine, GSK is taking steps toward expanding its label for Arexvy. The FDA has already endorsed it for people 60 and older. The results could pave the way for the shot to be approved for this age group.

pharmaphorum
AUGUST 25, 2021
Just a month after getting approval for its new pneumococcal vaccine Vaxneuvance in adults, Merck & Co has reported positive trial results in children that will ramp up the pressure on Pfizer and its market-leading Prevnar franchise.
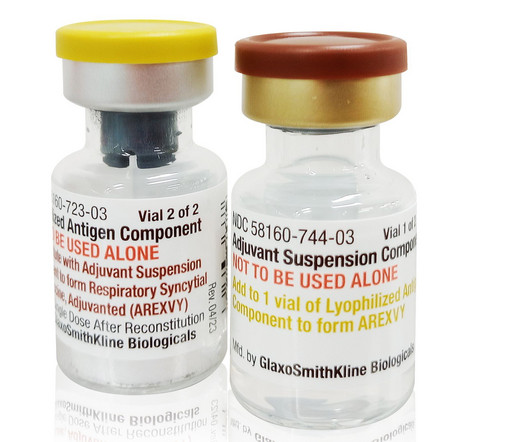
Fierce Pharma
JUNE 7, 2024
Thanks to respiratory syncytial virus (RSV) shot Arexvy’s new label expansion into a slightly younger pool of adults, GSK is more confident than ever that its vaccine will be able to conquer the co | The FDA approved Arexvy for RSV prevention in adults ages 50 to 59 who are at increased risk of developing RSV disease.

Pharmaceutical Technology
APRIL 24, 2023
Since then, the field of nanomedicine has steadily progressed to reach high points such as the successful use of nanotechnology to deliver messenger RNA (mRNA)-based Covid-19 vaccines. In the case of most mRNA vaccines, a lipid nanoparticle-based approach was chosen due to its ability to protect the mRNA in the body and prevent degradation.
pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford-AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.
pharmaphorum
JANUARY 8, 2021
As COVID-19 vaccines are hastily deployed in the UK for priority groups, a debate rages over the government’s controversial strategy to delay time between vaccine doses. When the UK announced the approval of the Pfizer-BioNTech and Oxford/AstraZeneca COVID-19 vaccines, it marked an exciting moment for the nation.

The FDA Law Blog
DECEMBER 3, 2023
The manufacturer’s agreement must cover all its labeler codes that contain an applicable drug or a selected drug. All labeler codes that are covered by Discount Program agreements will be distributed to PDP sponsors and posted on the CMS website. covered insulin product or vaccine). state pharmaceutical assistance programs).

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

The Checkup by Singlecare
JULY 8, 2024
The Food & Drug Administration (FDA) has approved Rinvoq to treat: Atopic dermatitis Rheumatoid arthritis Psoriatic arthritis Ulcerative colitis Crohn’s disease Ankylosing spondylitis Non-radiographic axial spondyloarthritis This article will unpack the benefits, dosage, side effects, and risks of Rinvoq for atopic dermatitis.

The Checkup by Singlecare
MAY 16, 2024
Understanding Remicade: Uses and mechanisms Remicade was the first tumor necrosis factor-alpha (TNF-alpha) inhibitor to be approved by the Food and Drug Administration (FDA). Since then, Remicade has also gained FDA indications for psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.

Pharmaceutical Technology
JULY 29, 2022
The Food and Drug Administration (FDA) has acc epted to review ImmunityBio’s Biologics License Application (BLA) for N-803 to treat Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients with or without Ta or T1 disease.

The Checkup by Singlecare
MAY 24, 2024
Its use in cats is “off-label,” meaning that the U.S. Food and Drug Administration ( FDA ) has not approved the drug for use in companion animals. Treatment lasts only a few days, with relatively minimal potential side effects. When cats have problems, they usually involve gastrointestinal issues like nausea or diarrhea.

pharmaphorum
MAY 17, 2021
Apellis Pharma has secured FDA approval for its complement C3 inhibitor Empaveli as a treatment for paroxysmal nocturnal hemoglobinuria (PNH) – with a label that will allow it to challenge Alexion’s established therapies directly. . The post Apellis set to take on Alexion as FDA clears PNH drug Empaveli appeared first on.

pharmaphorum
FEBRUARY 7, 2022
Sanofi has won FDA approval for its sutimlimab for cold agglutinin disease (CAD) at the second time of asking, becoming the first approved therapy for the rare blood disorder in the US. It also abandoned an mRNA-based vaccine for COVID-19 and suffered delays to another COVID-19 shot partnered with GlaxoSmithKline.
The Checkup by Singlecare
SEPTEMBER 13, 2024
The Food and Drug Administration (FDA) has approved Fasenra for maintenance treatment of severe asthma. Nucala has a more robust array of indications and has been FDA approved to treat severe eosinophilic asthma, eosinophilic granulomatosis with polyangiitis (EGPA), hypereosinophilic conditions, and rhinosinusitis with nasal polyps.
The Checkup by Singlecare
JULY 11, 2024
The drug’s label recommends doses of 5–60 mg per day. Food and Drug Administration ( FDA ), some common side effects include: Headache Nausea Increased appetite Trouble sleeping Weight gain Fatigue Fluid retention Acne Prednisone may also cause certain serious side effects. Is colchicine or prednisone better for gout?
The Checkup by Singlecare
APRIL 3, 2024
This can reduce stomach upset unless your medication is labeled “enteric coated” or “gastro-resistant.” Keep up-to-date on vaccines as recommended by your provider. Herbal supplements are not regulated by the FDA, so the full extent of interactions with drugs like prednisone is unknown.

pharmaphorum
OCTOBER 23, 2024
A new FDA approval has given Pfizer a broader label for its respiratory syncytial virus (RSV) vaccine Abrysvo than rival shots from GSK and Moderna, but it may not make much of a difference in the battle for market share.The US regulator has cleared Abrysvo for use in adults aged 18 to 59 at risk of RSV-related disease, extending its earlier label (..)
The Checkup by Singlecare
JULY 12, 2024
Beyond that overlap, Xolair is approved by the Food and Drug Administration (FDA) to treat chronic spontaneous urticaria (CSU)—previously referred to as chronic idiopathic urticaria (CIU)—the medical term for hives with no cause. In 2024, the FDA also approved Xolair for accidental exposure in people with food allergies.

The Checkup by Singlecare
OCTOBER 30, 2023
Food and Drug Administration (FDA) to treat a wide range of respiratory bacterial infections, such as pneumonia and bronchitis , as well as infections of the ear , nose, throat , urinary tract infections , and skin. Amoxicillin food interactions According to the amoxicillin product labeling , there are no drug-food interactions of concern.

PharmaShots
FEBRUARY 21, 2023
In Aug’21, the US FDA cleared QSam’s IND application for CycloSam. FAP-2286 (labeled with lutetium-177), a peptide-targeted radionuclide therapy (PTRT) and imaging agent targeting fibroblast activation protein (FAP), is the company’s lead asset amongst radiopharma products.

The Checkup by Singlecare
MAY 8, 2024
Common side effects of Remicade include: Mild infusion reactions Nausea or abdominal pain Headache Rash or itching Joint pain Fever Fatigue Adverse effects of Remicade can be much more severe, though—severe enough for the Food and Drug Administration (FDA) to issue multiple boxed warnings about the drug and its TNF-alpha blocker brethren.
ISPE
AUGUST 30, 2022
Recent US FDA data show that the lack of raw material availability contributes to 27% of drug shortages (see Appendix ). It is considered a moderate change by the FDA (CBE30) and NMPA. This change ranges from a moderate change (CBE-30) by the FDA to a minor change not requiring prior approval by the WHO.

The FDA Law Blog
JANUARY 3, 2024
Enter the FDA’s Center for Veterinary Medicine (“CVM”). A longstanding component of the FDA and its predecessor, CVM, in its initial form as part of the Department of Health, Education, and Welfare, was established in 1965 and evolved in CVM by 1984. And CVM certainly exercises that authority.
The Checkup by Singlecare
SEPTEMBER 13, 2024
Stelara (ustekinumab) is an injectable drug approved by the Food and Drug Administration (FDA) for the treatment of autoimmune conditions, such as plaque psoriasis, active psoriatic arthritis, and inflammatory bowel diseases, including Crohn’s disease , and ulcerative colitis. There are no known food-drug interactions with Stelara.

Pharmaceutical Technology
NOVEMBER 30, 2022
On September 16, another rare disease therapy, bluebird bio’s Skysona (elivaldogene autotemcel), received FDA approval to treat early, active cerebral adrenoleukodystrophy (CALD) in boys between the ages of four and 17. Source: GlobalData Pharmaceutical Intelligence Center.

pharmaphorum
JANUARY 12, 2023
The label for Xofluza (baloxavir marboxil) has been extended to include the treatment of uncomplicated flu infections in children aged one year and over, a particularly vulnerable group of patients, as well as post-exposure prophylaxis in those who are in close contact with an infected individual.

Pharmaceutical Technology
MARCH 21, 2023
FDA and EMA decisions In January, the European Commission (EC) approved Roche’s Xofluza (baloxavir marboxil) to prevent and treat influenza in children ages one year and older. A few weeks later, the EC extended the approval label of AstraZeneca’s Forxiga (dapagliflozin) to include the treatment of symptomatic hearth failure.
The Checkup by Singlecare
SEPTEMBER 12, 2024
Both are brand-name drugs approved by the Food and Drug Administration (FDA) and are given as subcutaneous injections. They also have different FDA approvals for some conditions. Enbrel is FDA approved for adults with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.

pharmaphorum
MARCH 18, 2022
Moderna has asked the FDA for emergency use authorisation for a fourth dose of its mRNA COVID-19 vaccine SpikeVax, following in the footsteps of Pfizer/BioNTech, which filed their Comirnaty shot earlier this week. There’s no guidance yet from the CDC or FDA on whether a second booster will be needed.
The Checkup by Singlecare
NOVEMBER 30, 2023
Acetaminophen may also blunt the effects of other medications that impact the immune system, including some medicines used to manage cancer diagnoses and even the immune response following vaccinations. In some cases, metyrapone is used off-label in the treatment of Cushing syndrome.

The FDA Law Blog
JUNE 2, 2023
“All In” Manufacturer Definition CMS believes that the National Rebate Agreement (NRA) requires a “manufacturer” to report to Medicaid all of its covered outpatient drugs, under all of its labeler codes. This includes newly acquired labeler codes and newly formed subsidiaries. 42 U.S.C. §§ 1396r-8(k)(3) and 1396r-8(g)(1)(B)(i).

PharmaShots
MARCH 9, 2023
Since its launch in 2016, Tivic Health has obtained three regulatory clearances for ClearUP, including FDA 510(k), FDA De Novo, and CE Mark, four patents, and numerous awards. Designation: Global Head Previous company: Merck Global Vaccines (2 yrs. Present company and time period: Labcorp (32 yrs.

The Checkup by Singlecare
OCTOBER 10, 2023
Cephalexin and alcohol Product labeling does not advise alcohol avoidance with cephalexin. Cephalexin and probiotics or oral live vaccines This one makes perfect sense. Oral live vaccines contain weakened bacteria, such as typhoid and cholera. Oral live vaccines contain weakened bacteria, such as typhoid and cholera.

PharmExec
JANUARY 9, 2025
Abrysvo and Arexvy will now be required to come with labeling that includes a warning about a potential increased risk of Guillain-Barr Syndrome.

Pharmaceutical Technology
OCTOBER 23, 2024
Abrysvo is now approved to prevent lower respiratory tract disease caused by RSV in high-risk adults over 18 years of age.
European Pharmaceutical Review
SEPTEMBER 22, 2023
Limitations of monoclonal antibody therapies Regulatory approvals from the US Food and Drug Administration (FDA) for aducanumab and lecanemab – and likely very soon for donanemab also – opened a route for different therapeutic modalities and other relevant disease targets, such as tau.

PharmaShots
APRIL 13, 2023
Its subsidiary, DePuy's CHARITE Artificial Disc for degenerative disc disease was the first device to be approved by the US FDA By acquiring Roche’s OTC business, Bayer was able to strengthen its consumer health business. Its top 8 products contributed more than $1B to the company’s net revenue. compared to 2015.

Fierce Pharma
MAY 1, 2024
Even with the threat of a potential new competitor in the respiratory syncytial virus (RSV) vaccine race on the horizon, GSK and its leading Arexvy shot aren’t sweating. | The company's respiratory syncytial virus vaccine Arexvy takes some two-thirds of the RSV market share, GSK reported. adults to its reach.

pharmaphorum
MARCH 16, 2022
Earlier this week, Pfizer chief executive Albert Bourla said that a second booster of its COVID-19 vaccine will be necessary to keep the pandemic under control, and the company has now asked the FDA to back this use. ” The post Pfizer makes case for fourth COVID jab dose to FDA appeared first on.

European Pharmaceutical Review
SEPTEMBER 12, 2022
The first aims to explore whether intradermal delivery of the Jynneos monkeypox vaccine is efficacious, and could thereby be used to expand the limited vaccine supply ; while the second aims to establish the efficacy of SIGA’s antiviral tecovirimat (TPOXX). Intradermal vaccine delivery. Exploring antiviral efficacy.
Pharmaceutical Technology
JULY 14, 2022
This analysis covers late April to May and is based on a list of CMOs impacted by regulatory decisions by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and reimbursement authorities like the UK’s National Institute of Health and Care Excellence (NICE). Covid-19 vaccines stay in the spotlight.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content