FDA Proposes Front-of-Package Nutrition Label for Most Packaged Foods
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmacy Times
JANUARY 17, 2025
If finalized, the requirement would include readily available nutrition information, including saturated fat, sodium, and added sugar content.

STAT
JUNE 20, 2023
WASHINGTON — The FDA wants to make it easier for consumers to know if the foods they’re buying are unhealthy — but doing so is harder than it seems here in the United States. Now, the Food and Drug Administration is embarking on a major study to test front of package labels here in the U.S.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
OCTOBER 7, 2022
The FDA has announced the set of rules it proposes to enforce for manufacturers to claim that a food product is “healthy.” ” The proposed rules are a lot better than the labeling anarchy that currently exists. But here’s my bottom line: health claims are not about health.

Pharmafile
APRIL 14, 2023
Pharma giant Eli Lilly’s Biologic Licence Application (BLA) for its bowel disease drug has been declined by the FDA over concerns about the proposed manufacturing of the drug. However, concerns weren’t expressed over the clinical data package, safety or label for the medicine.

STAT
AUGUST 21, 2023
A 2019 policy requires companies that make unhealthy foods to include warning labels on the front of any boxes they sell in Mexico to educate consumers about things like excess sugar and fat. MEXICO CITY — Kellogg’s is waging a war here over Tigre Toño and Sam el Tucán. Read the rest…

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g., 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g.,

European Pharmaceutical Review
AUGUST 23, 2022
A study of the causes of warning letters issued by the US Food and Drug Administration (FDA)’s Center for Drug Evaluation and Research (CDER) and Center for Devices and Radiological Health (CDRH) between 2010 and 2020 revealed that poor current good manufacturing practice (cGMP) compliance and misbranding were the most common citations.

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. Ophthalmic drug products should be evaluated for extractables and leachables, FDA asserted.

STAT
OCTOBER 19, 2022
After years of deliberation, the FDA recently announced a new set of rules it proposes to regulate claims on food packaging that a product is “healthy.” ” The most basic rule: the product must actually contain food, not just ingredients. Read the rest…

Roots Analysis
APRIL 20, 2022
On an average, around 50 drugs are approved by the US Food and Drug Administration (US FDA) annually. This continuously growing pipeline of pharmaceutical drug products has inadvertently led to an increase in the demand for their associated primary packaging and Secondary Packaging solutions. . trillion in 2023.

The FDA Law Blog
FEBRUARY 21, 2024
Mullen — More than five years after FDA first announced its plan to harmonize 21 CFR Part 820 with ISO 13485, on February 2, 2024, FDA finally issued the Quality Management System Regulation (QMSR) Final Rule. FDA further retained some definitions in the QSMR. Notably, Part 820 will look different. Revised § 820.3

Pharmaceutical Technology
APRIL 26, 2023
Packaging plays a critical part in the pharmaceuticals and medical devices industries and is developed with its own set of security standards for the safety of consumers. The role of primary packaging has extended beyond the primary objectives of sterility, physical and chemical protection, and security.

Roots Analysis
AUGUST 26, 2024
In today’s global landscape, sustainability has emerged as a pivotal concern across various sectors including the pharmaceutical packaging industry. What is Sustainable Packaging / Green Packaging? The following figure presents the raw materials used by the sustainable packaging companies.

The FDA Law Blog
AUGUST 17, 2023
FDA-2023-P-0313 and FDA-2023-P-0344 ) regarding its product Hetlioz (tasimelteon). Vanda requested that FDA revoke the approval of Apotex’s and Teva’s generic versions of Hetlioz on the grounds that the generic tasimelteon products did not meet the statutory “same labeling” requirement for generic drugs found in 21 U.S.C. §

The FDA Law Blog
JULY 10, 2023
After a firm submits a 510(k) to FDA, FDA will request still more information after a first-pass review. According to the 2 nd Quarter FY2023 MDUFA V Performance Report , FDA issued a request for additional information (AI request) on the first FDA review cycle for 63% to 68% of 510(k)s submitted in FY2018 to FY2022.

The FDA Law Blog
SEPTEMBER 29, 2022
The statute itself – the National Bioengineered Food Disclosure Standard – requires use of one of three forms of disclosure: on-package text, a symbol, or an electronic or digital link. Thus, the Court concluded that addition of the standalone text message disclosure options was inconsistent with the statute’s mandate.

The FDA Law Blog
NOVEMBER 14, 2022
2022, FDA published a draft guidance on FDA’s implementation of the Over-the-Counter Monograph Drug User Fee Program (OMUFA). FDA is authorized to charge an annual facility fee for OTC monograph drug facilities. FDA will not refund any fees that have been incurred already). By Riëtte van Laack & Deborah L.

Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has rejected Eli Lilly’s biologic licence application (BLA) for the ulcerative colitis (UC) drug mirikizumab over manufacturing concerns. No concerns related to the clinical data package, safety or the medicine label. The regulator has issued a complete response letter.

The FDA Law Blog
MARCH 27, 2023
Such codes need to be placed on device labels and packages to allow devices to be easily identified and tracked throughout their lifecycle, except where the rule provided for an exception or alternative. Devices can have both a UPC code and a UDI on their label and package. Most of the compliance dates have been passed.

The FDA Law Blog
JUNE 26, 2024
Lenz, Principal Medical Device Regulation Expert — FDA recently released a new eSTAR template for device pre-submissions and 513(g) Requests for Information, referred to as PreSTAR. A pre-submission provides the submitter an opportunity to obtain FDA feedback prior to a planned medical device premarket submission.

The FDA Law Blog
JULY 22, 2024
FDA Law Blog readers receive a 10% discount off the tuition fee (promo code D10-999-FDA25 ). s John W.M. Claud will be speaking at the conference in a session titled “Crafting Your Safety Blueprint for Adverse Events and Recalls under MoCRA.” You can register for the conference here.

The Checkup by Singlecare
JULY 22, 2024
It can also treat parasitic infections, like giardia, and is frequently used off-label (for a non-FDA-approved use) to help treat Crohn’s disease, bite wounds, and oral infections. Be sure to check food labels carefully for either of these ingredients. What should you eat while taking metronidazole?

pharmaphorum
OCTOBER 1, 2020
Shares in US biotech CTI BioPharma have shot up after the FDA agreed to an accelerated review early next year of its lead drug pacritinib for low blood platelets (thrombocytopenia) caused by myelofibrosis. The post CTI wins over FDA to claim early review of myelofibrosis drug appeared first on. after the announcement.

The FDA Law Blog
OCTOBER 5, 2022
By Riëtte van Laack — On September 28, 2022, FDA announced the availability of the proposed rule for the implied nutrient content claim “healthy.” The term healthy, as an implied nutrient claim, was first defined by FDA in 1994. FDA also announced it would be re-evaluating the regulatory criteria for use of the “healthy” claim.

Pharmaceutical Technology
APRIL 21, 2023
On 14 April, the FDA rejected Lilly’s biologic licence application (BLA) for their anti-interleukin (IL)-23, mirikizumab, which is in development for the treatment of ulcerative colitis (UC). The recent setback dents mirikizumab’s chances to be the first among the IL-23 inhibitors to launch in the US for UC.
The Checkup by Singlecare
JUNE 17, 2024
It’s also can be used off-label to treat morning sickness during pregnancy. Zofran vs. ondansetron The FDA withdrew Zofran oral dissolving tablets (ODT) from the market in 2023, but not due to safety or effectiveness; instead, the decision was related to packaging concerns, says Dr. Glatter. Consult a healthcare professional.

The FDA Law Blog
SEPTEMBER 6, 2023
Food and Drug Administration – labeled an “Official Action Indicated” classification – is generally devastating for the facility, not least because it can stall FDA approval of applications to market drugs manufactured at the facility. And the Guidance may be used as leverage to secure action from FDA on a meeting request.

Pharma Marketing Network
AUGUST 9, 2023
It’s crucial for marketing teams to stay informed about the latest guidelines, policies, and updates from regulatory bodies such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in the European Union. Keep thorough records of approvals for future reference.

The Checkup by Singlecare
MARCH 22, 2023
We have a regulator called Health Canada, similar to the Food and Drug Administration (FDA), which works to ensure the safety of our drugs,” Tadrous says. The FDA allows customers to purchase drugs from Canadian online pharmacies and have them shipped to the U.S. If you don’t live near the border between the U.S. Are not licensed.

The FDA Law Blog
JUNE 12, 2023
By Riëtte van Laack — FDA regulates pet food similar to other animal foods. The Federal Food, Drug, and Cosmetic Act (FDC Act) requires that all animal foods, like human foods, be safe to eat, produced under sanitary conditions, contain no harmful substances, and be truthfully labeled.

The Checkup by Singlecare
AUGUST 8, 2024
She also explains that “Zyrtec’s use for dogs is ‘ off-label.'” Check the drug’s packaging and label carefully before giving it to your pet. The FDA says Zyrtec’s effects typically last around 24 hours for humans, and studies show that its elimination half-life is around 8.3

The Checkup by Singlecare
JANUARY 4, 2024
Always follow the directions on the packaging or consult a healthcare professional if you have concerns about the appropriate dosage. In addition, the FDA label for the nighttime forms of Mucinex containing acetaminophen recommends avoiding three or more drinks per day.

The People's Pharmacy
JUNE 12, 2023
If a patient wants to report an adverse reaction to the FDA, they need to fill out Form 3500B. It is rare for pharmacies to put the expiration date on the label if they transfer the pills from the manufacturer’s original container. After all, computers easily generate a date one year from the dispensing day when printing the label.

The Checkup by Singlecare
DECEMBER 29, 2023
Because Zyrtec causes sleepiness, the most concerning drug interactions involve drugs that also cause sedation, such as: Other antihistamines Tranquilizers Opioids Benzodiazepines Gabapentin General anesthetics Alcohol Cannabis Safety measures while using Zyrtec What are the FDA warnings about Zyrtec? Who should never take Zyrtec?
The Checkup by Singlecare
DECEMBER 2, 2024
Your Zyrtec will expire, and the Food and Drug Administration (FDA) requires the manufacturer to include the expiration date on the product. Drugs are chemical compounds that can break down over time, and the FDA wants to ensure that consumers are taking safe and effective medication. Knowing more can help you better prepare.
The Checkup by Singlecare
MARCH 21, 2024
Nonetheless, healthcare providers may prescribe it off-label for weight loss. Its active ingredient, semaglutide, is FDA approved for weight loss under the brand name Wegovy , which has a dosing progression of 0.25 Ozempic can be effective across all dosages for weight loss, but higher doses saw better results. mg, 1 mg, 1.7

pharmaphorum
JANUARY 30, 2023
For its part, the US Food and Drug Administration (FDA) posted its drug shortage list , which contains various formulations of amoxicillin that are limited in supply. Looking to the long-term However, despite the issue being exacerbated by geopolitical factors, the challenge is a broad one that requires a long-term strategy to navigate.
Pharmaceutical Technology
JUNE 20, 2023
UK-based biotech F2G was dealt a blow as the US’ FDA issued a complete response letter rejecting its latest new drug application (NDA). F2G claimed in a June statement that the FDA requested additional data and analysis that extended beyond the current review period.

The Checkup by Singlecare
JULY 30, 2024
Food and Drug Administration ( FDA ) to treat pain and fever. Zyrtec (cetirizine) is an antihistamine approved by the FDA to treat allergies with respiratory symptoms, like runny nose, sneezing, watery eyes, itchy eyes, and itching in the nose and throat. Always read labels and instructions carefully.

Pharma Marketing Network
AUGUST 9, 2023
It’s crucial for marketing teams to stay informed about the latest guidelines, policies, and updates from regulatory bodies such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in the European Union. Keep thorough records of approvals for future reference.

The Checkup by Singlecare
FEBRUARY 8, 2024
Food and Drug Administration (FDA) advisory committee met to discuss evidence suggesting that oral phenylephrine, one of the active ingredients found in most over-the-counter decongestants, is ineffective for relieving nasal congestion. However, the FDA has not yet arrived at a definite conclusion as the evidence is still under review.
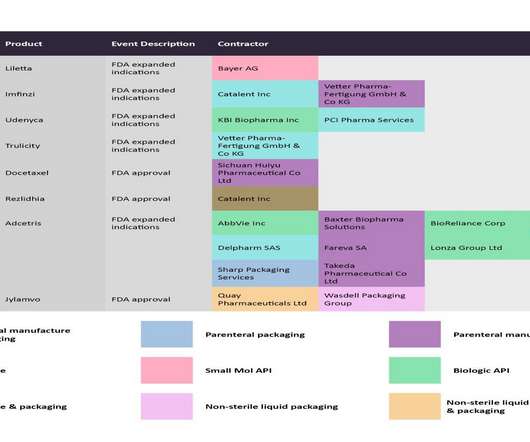
Pharmaceutical Technology
JANUARY 15, 2023
While Catalent Inc and PCI Pharma Services are in charge of the solid dose and packaging of Lupkynis, Lonza is manufacturing the small molecule API. In the same month, the EMA expanded the label of Gilead Sciences’ Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide) to include its use for virologically suppressed HIV-positive children.
The Checkup by Singlecare
JULY 17, 2023
The FDA has approved OTC docusate as an initial treatment of occasional constipation, so healthcare professionals commonly prescribe or recommend docusate. Docusate package instructions generally advise taking the laxative no longer than a week. A 238-gram package of MiraLAX powder costs about $17.

The FDA Law Blog
NOVEMBER 2, 2023
Lewis, Senior Regulatory Device & Biologics Expert — On October 20, 2023, FDA announced the availability of the final guidance authored by CBER titled “Voluntary Consensus Standards Recognition Program for Regenerative Medicine Therapies.” Existing published VCS may be identified internally by FDA or externally by stakeholders.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content