Immunization Roundup: FDA Approves FluMist, Updated Novavax COVID-19 Vaccine
Drug Topics
SEPTEMBER 25, 2024
Catch up on important immunization news from the month of September.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Drug Topics
SEPTEMBER 25, 2024
Catch up on important immunization news from the month of September.
Drug Topics
AUGUST 2, 2024
The technology from Diakonos Oncology initiates a natural immune response that targets and eliminates cancer cells by activating cytotoxic TH1 cell signaling pathways.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Topics
MARCH 27, 2023
The drug Leniolisib, now with the brand name Joenja, treats APDA, a genetic disorder that impairs the immune system. Its annual list price will be $547,500.

STAT
MARCH 22, 2024
The Food and Drug Administration on Friday authorized a new antibody to protect immunocompromised individuals against Covid-19. The drug, known as Pemgarda and marketed by the biotech Invivyd, is the first such drug to become available since the agency pulled AstraZeneca’s Evusheld off the market in January 2023.
Pharmacy Times
SEPTEMBER 23, 2024
Approved with 3 new indications, bimekizumab-bkzx is the first and only interleukin-17 inhibitor approved for these diseases.

Pharmacy Times
JUNE 18, 2024
V116 (Capvaxive; Merck) elicited higher immune responses than the comparator for the serotypes that are unique to the vaccine, according to studies submitted to the FDA.
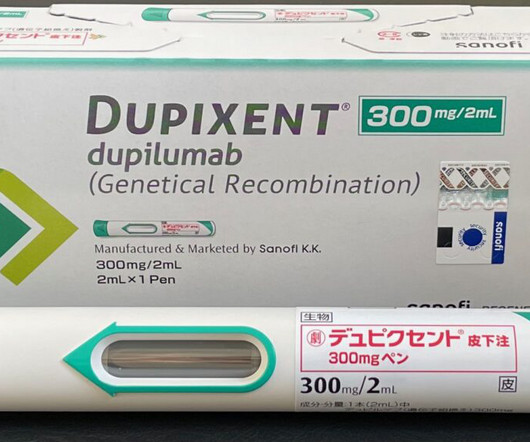
STAT
SEPTEMBER 27, 2024
Dupixent is already a blockbuster therapy for a range of immune-mediated diseases, but the Food and Drug Administration’s approval of the drug for chronic obstructive pulmonary disease greatly expands its reach — and could give it an edge over rival treatments in development. There are 300,000 such people in the U.S.,

Pharmacy Times
OCTOBER 1, 2024
Ustekinumab is a human monoclonal antibody targeting the cytokines interleukin (IL)-12 and IL-23, which play a rule in the inflammatory and immune responses.

Pharmacy Times
APRIL 17, 2024
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.

Fierce Pharma
OCTOBER 16, 2023
Up until this point, immune checkpoint inhibitors have been allowed to treat early-stage non-small cell lung cancer (NSCLC) either before or after surgery. Thanks to a new FDA approval for Merck's Keytruda, a continuous immunotherapy regimen around surgery is now available for patients with early-stage non-small cell lung cancer.

Fierce Pharma
JANUARY 16, 2024
The FDA has approved Takeda's HyQvia as a maintenance therapy for CIDP. It is the second indication for HyQvia, which was first endorsed in 2014.

Pharmacy Times
SEPTEMBER 18, 2024
Eosinophilic granulomatosis with polyangiitis is a rare and immune-mediate vasculitis that can damage multiple organs and be fatal if left untreated.
Pharmacy Times
JULY 12, 2024
RSVpreF is currently the only FDA-approved RSV vaccine for pregnant individuals.

Hospital Pharmacy Europe
JULY 17, 2023
Immune checkpoint inhibitors have transformed the management of non-small cell lung cancer, but how long should treatment be continued for optimal survival? It has long been recognised that a hallmark of cancer is immune evasion and that the immune system is held back by inhibitory immune checkpoint receptors and ligands.

Pharmacy Times
AUGUST 16, 2024
Durvalumab is a human monoclonal antibody that targets and binds to PD-L1 to interrupt tumor immune-evasion tactics.

Pharmaceutical Technology
JULY 25, 2024
JNJ-2056 is being investigated in the Phase IIb ReTain study which enrols participants who are yet to show Alzheimer’s symptoms.

Pharmafile
APRIL 14, 2023
Pharma giant Eli Lilly’s Biologic Licence Application (BLA) for its bowel disease drug has been declined by the FDA over concerns about the proposed manufacturing of the drug. However, concerns weren’t expressed over the clinical data package, safety or label for the medicine.
Pharmacy Times
SEPTEMBER 20, 2024
Amivatamab-vmjw is a fully-human bispecific antibody targeting EGFR and MET with immune cell-directed activity.

STAT
JUNE 28, 2024
The New York-based biotech said the FDA asked for only “limited” additional information. ” The rejection is another setback for a class of gene therapies that have held tremendous promise for patients with rare blood and immune disorders but have proven exceptionally difficult to manufacture and scale.

Pharmacy Times
MARCH 25, 2024
The indication is for adults and adolescents with moderate to severe immune compromise due to medical conditions or immunosuppressive medications and treatments.

STAT
AUGUST 21, 2023
The Food and Drug Administration on Monday approved a Pfizer vaccine that aims to protect newborns against RSV by vaccinating pregnant people in the latter part of pregnancy. The vaccine, Abrysvo, has also been approved for use in adults 60 and older to protect them against respiratory syncytial virus. Read the rest…

European Pharmaceutical Review
NOVEMBER 18, 2022
Tzield (teplizumab-mzwv), the first drug to help prolong the onset of stage 3 type 1 diabetes in adults and children over eight years old with stage 2 type 1 diabetes, has been approved by the US Food and Drug Administration (FDA). The drug binds to certain immune system cells and delays progression to stage 3 type 1 diabetes.

Pharmacy Times
SEPTEMBER 20, 2024
The approval enables more convenient flu immunization options for patients.

pharmaphorum
DECEMBER 19, 2022
The US Food and Drug Administration (FDA) has approved Swiss drugmaker Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firedenovec-vncg) for the treatment of adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumours.

The Checkup by Singlecare
NOVEMBER 6, 2023
Food and Drug Administration (FDA) just approved Wezlana (ustekinumab-auub), a biosimilar for the popular drug Stelara. According to the FDA announcement, the most serious side effect of Wezlana is infection because the prescription affects your immune response.

pharmaphorum
APRIL 22, 2022
Ampio Pharma’s candidate therapy for knee osteoarthritis has been knocked back by the FDA, which will likely now require a new clinical trial of the drug before it will consider a review. The FDA said it should have been advised of the proposed changes before Ampio unblinded and analysed the data from the AP-013 study.

STAT
FEBRUARY 16, 2024
Nearly four decades after its first conception, the first TIL therapy, an immunotherapy that harvests cancer-fighting immune cells from the patient’s own body, received accelerated approval from the Food and Drug Administration for advanced melanoma.

Fierce Pharma
OCTOBER 20, 2023
approval for the first five-pronged meningitis vaccine, Pfizer has eked out a regulatory win well ahead of its immunization rival GSK. | FDA gave a thumbs-up to Penbraya, the commercial moniker for Pfizer’s pentavalent vaccine defending against the most common serogroups behind meningococcal disease. In the race to secure U.S.
Pharmacy Times
JUNE 23, 2022
Vaxneuvance was approved for an expanded indication by the FDA on June 22, 2022, for active immunization in children 6 weeks of age and older.

pharmaphorum
DECEMBER 30, 2021
Johnson & Johnson’s much-touted crop of bispecific antibodies for cancer generated its first commercial product in May, and the drugmaker has now filed for FDA approval of a second candidate. “We look forward to working with the FDA in their review of our teclistamab submission.”
European Pharmaceutical Review
AUGUST 2, 2024
The US Food and Drug Administration (FDA) has granted accelerated approval for the cell therapy TECELRA ® (afamitresgene autoleucel) to treat adults with unresectable or metastatic forms of the disease. The post FDA approves innovative engineered cell therapy appeared first on European Pharmaceutical Review.

European Pharmaceutical Review
DECEMBER 19, 2022
The US Food and Drug Administration (FDA) has approved Adstiladrin (nadofaragene firadenovec-vncg) as the first gene therapy for non-muscle-invasive bladder cancer (NMIBC) in adults with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive with carcinoma in situ (CIS) with or without papillary tumours.

pharmaphorum
DECEMBER 15, 2021
Bristol-Myers Squibb’s rheumatoid arthritis drug Orencia has been approved by the FDA to prevent graft versus host disease (GvHD), a serious complication of haematopoietic stem cell transplant (HSCT) used to treat leukaemias and other blood cancers. billion in the first nine months of the year.

The Checkup by Singlecare
NOVEMBER 23, 2022
Food and Drug Administration (FDA) approved the first drug to delay the onset of Type 1 diabetes. Tzield received priority review from the FDA, a status given to drugs with the potential for “significant improvement” in the treatment, diagnosis, or prevention of a serious condition. 17, the U.S. That’s where Tzield comes in.

Fierce Pharma
MARCH 25, 2024
The four years since the start of the COVID-19 pandemic have yielded many advances against the coronavirus, including Moderna and Pfizer’s groundbreaking mRNA vaccines. |

European Pharmaceutical Review
NOVEMBER 28, 2022
Fast Track designation (FTD) has been granted for REM-001 therapy to treat unresectable cutaneous metastatic breast cancer (CMBC) by the US Food and Drug Administration (FDA). The post FDA Fast Track designation for photodynamic cancer therapy appeared first on European Pharmaceutical Review.

pharmaphorum
OCTOBER 21, 2021
The FDA has authorised booster shots with Moderna and Johnson & Johnson’s COVID-19 vaccines, a month after giving the go-ahead to a third-dose of Pfizer/BioNTech’s shot. The FDA has followed that advice. The post FDA okays Moderna, J&J COVID jab boosters, plus ‘mix and match’ appeared first on.

Pharmacy Times
JUNE 16, 2023
Glofitamab targets CD3, a protein found on the surface of immune T cells in patients with relapsed or refractory diffuse large B-cell lymphoma, and CD20, a healthy or malignant protein that lines the surfaces of B cells.
The Checkup by Singlecare
DECEMBER 26, 2024
Over 40 new medications have been approved by the FDA, giving patients more options for treating various health problems. Zelsuvmi (berdazimer) Zelsuvmi received the green light from the FDA on January 5, 2024. Zelsuvmi’s approval is a big deal because it’s only the second FDA-approved treatment for this condition.

STAT
OCTOBER 8, 2024
Moreover, the trade group maintained the FDA move was “unlawful,” because it failed to follow so-called rule-making procedures and provide proper notice of its plans. In the late 1990s, the FDA raised concerns the vaccine lost potency toward the end of its 24-month shelf life. ” A U.S. from 1967 until 2022.

Pharmaceutical Technology
JULY 29, 2022
The Food and Drug Administration (FDA) has acc epted to review ImmunityBio’s Biologics License Application (BLA) for N-803 to treat Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients with or without Ta or T1 disease.

Pharmaceutical Technology
FEBRUARY 20, 2023
The US Food and Drug Administration (FDA) has granted approval for Apellis Pharmaceuticals’ Syfovre (pegcetacoplan injection) to treat geographic atrophy (GA), an advanced form of age-related macular degeneration (AMD). The post US FDA approves Apellis’ geographic atrophy therapy Syfovre appeared first on Pharmaceutical Technology.

pharmaphorum
JUNE 10, 2022
The FDA may have safety concerns abut bluebird bio’s gene therapy for rare, fatal disease cerebral adrenoleukodystrophy (CALD), but its advisors believe its benefits far outweigh the risks. The post FDA panel backs bluebird’s CALD gene therapy, despite safety worries appeared first on.
Digital Pharmacist
OCTOBER 26, 2022
Food and Drug Administration (FDA) announced its approval of the Boostrix vaccine, commonly known as Tdap (combination of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis) for immunization administration during the third trimester of pregnancy to prevent pertussis in infants younger than two months of age.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content