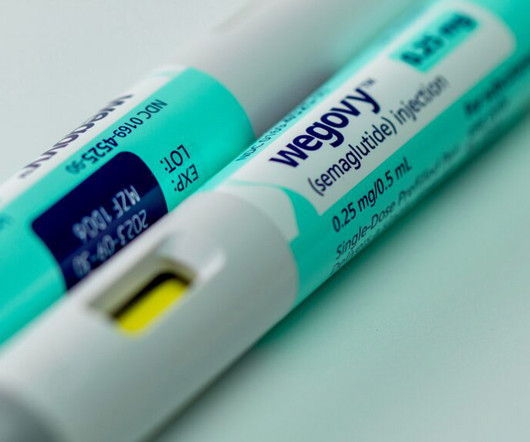
FDA Adds Delayed Gastric Emptying as Adverse Event on Semaglutide Label
Pharmacy Times
NOVEMBER 14, 2024
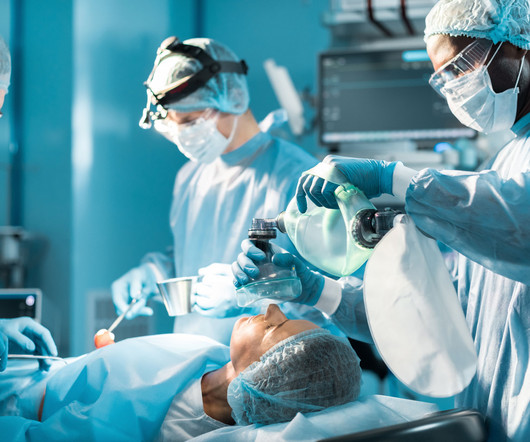
The label includes postmarking reports showing rare instances of pulmonary aspiration for patients undergoing procedures that require general anesthesia or deep sedation.














































Let's personalize your content