Inventing the COVID vax pill: a matter of convenience and durability
PharmaVoice
AUGUST 17, 2022
Recent data from Vaxart’s phase 1 trial of its COVID-19 inoculation pill shows potential.

PharmaVoice
AUGUST 17, 2022
Recent data from Vaxart’s phase 1 trial of its COVID-19 inoculation pill shows potential.

PhRMA
AUGUST 16, 2022
Innovative biopharmaceutical research companies have been dedicated to fighting COVID-19 for over two years. Since the start of the pandemic, America’s biopharmaceutical companies have worked around the clock to research, develop and manufacture treatments and vaccines to fight COVID-19. We’ve made unprecedented progress in a short time thanks to decades of experience having produced over 14 billion vaccines globally to date.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmacy Times
AUGUST 18, 2022
Analysis is based on functional magnetic resonance imaging to provide insight into the effective connectivity.

IDStewardship
AUGUST 17, 2022
In this article tips for running successful meetings as a pharmacist are provided and discussed. . Authored By: Timothy P. Gauthier, Pharm.D., BCPS, BCIDP. Article posted 21 August 2022. Meetings are a part of life in the professional world, particularly for pharmacists that work in administrative, clinical, or industry positions. This includes having internal pharmacy department meetings as well as coordinating inter-professional institutional meetings such as a Pharmacy and Therapeutics Commit

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

PharmaVoice
AUGUST 16, 2022
Nasal vaccines under development by Codagenix, Xanadu Bio and others have the potential to finally help reduce virus transmission and breakthrough infections.

PhRMA
AUGUST 15, 2022
With Congress sending to the president a partisan reconciliation spending bill, the cost of insulin is receiving greater attention. Numerous proposals have been introduced in recent months aimed at capping what patients pay out of pocket for insulin. Despite what the White House’s talking points falsely suggest , PhRMA hasn’t taken a position on these bills.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

European Pharmaceutical Review
AUGUST 17, 2022
The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) has granted marketing authorisation for Pluvicto ® (lutetium [ 177 Lu] vipivotide tetraxetan), for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.

PharmaVoice
AUGUST 18, 2022
Now that Medicare will be able to negotiate drug prices under the Inflation Reduction Act, the industry can expect to see some changes. Here are some tips on how to navigate the changing tide.

Pharmaceutical Technology
AUGUST 17, 2022
On August 8, Pfizer and Valneva announced the initiation of a Phase III study with their Lyme disease vaccine , bringing the prospect of an injection to prevent the condition disease one step closer to reality. VLA15 is the only vaccine for Lyme disease in clinical development, according to GlobalData, the parent company of Pharmaceutical Technology.

Pharmacy Times
AUGUST 17, 2022
Investigators from University of Texas Health Science Center at Houston analyze data from individuals aged 5 to 18 years enrolled in the Texas CARES survey.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Outsourcing Pharma
AUGUST 16, 2022
The AI and QC specialists have joined to use AI and QC in order to discover biological pathways relevant to the treatment of triple-negative breast cancer.

PharmaVoice
AUGUST 17, 2022
Upon joining Takeda Pharmaceuticals as president of the global oncology unit, Teresa Bitetti had two important goals: to transform the business and create greater synergies between commercial and R&D operations.

Pharma Tutor
AUGUST 14, 2022
A Saga of 75 years journey of Indian Pharmaceutical Industry. Look back of growth drive for Indian pharmaceutical industry after 75 years of independence and future roadmap. admin. Sun, 08/14/2022 - 18:21. Tags. Pharmapedia. Articles.

Pharmacy Times
AUGUST 17, 2022
Rajul A. Patel, PharmD, PhD, director of the Medicare Part D Outreach Clinics at the University of the Pacific in Stockton, California, discusses his work providing drug savings advice to Medicare beneficiaries in diverse communities.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

European Pharmaceutical Review
AUGUST 15, 2022
Research suggests copper (Cu) and silver (Ag) coated surfaces display significant differences in antiviral properties and that while copper is effective at reducing Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2; the cause of COVID-19) transmission, silver is not. As a result of corrosion, both copper and silver release positively charged ions into their environment, which can prevent bacterial growth or kill off the cells completely.

PharmaVoice
AUGUST 16, 2022
In balancing two roles — Lexitas CEO and practicing physician — George Magrath brings a patient-first perspective to developing and advancing eye treatments.
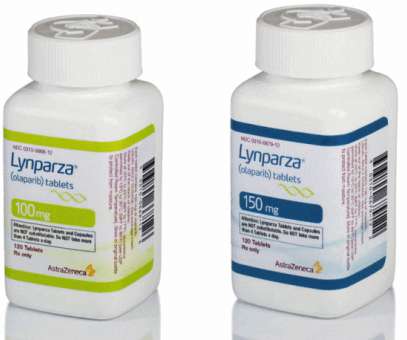
pharmaphorum
AUGUST 16, 2022
AstraZeneca and Merck & Co’s PARP inhibitor Lynparza is already used to treat prostate cancer associated with a specific genetic mutation, but could see its use broadened if a new marketing application is approved by the FDA. The US regulator has started a priority review of Lynparza (olaparib) in combination with abiraterone and prednisone or prednisolone as a first-line treatment for metastatic castration-resistant prostate cancer (mCRPC) based on the results of the PROpel trial.

Pharmacy Times
AUGUST 17, 2022
Drs Lodise and Feuerstadt discuss risk factors for C. difficile infection (CDI).

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

European Pharmaceutical Review
AUGUST 16, 2022
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved an updated version of Moderna’s COVID-19 vaccine, Spikevax, that targets two coronavirus variants as a booster. The regulator confirmed that the vaccine meets its standards of safety, quality and effectiveness. The decision was also endorsed by the government’s independent expert scientific advisory body, the Commission on Human Medicines, after a careful review of the evidence.

PharmaVoice
AUGUST 15, 2022
Analysts are watching how cultural and political debates around reproductive rights may impact HRA Pharma's OTC birth control application.

pharmaphorum
AUGUST 18, 2022
Endo International is the latest drugmaker to file for chapter 11 bankruptcy protection in connection with opioid litigation in the US, after agreeing a $6 billion deal with creditors that includes an offer to settle outstanding lawsuits. The Ireland-domiciled company is struggling under the weight of around $8 billion in debt, and has been hamstrung by costs associated with fighting thousands of suits that accuse it of wrongdoing in its marketing and promotion of painkiller Opana ER (oxymorphon

Pharmacy Times
AUGUST 15, 2022
Patients with gout have a 4 times greater risk of a cardiovascular episode within the 60 days following their flare up.

The Honest Apothecary
AUGUST 19, 2022
It is my conviction that managers really do make all the difference in the world. Great managers get great results and build great teams. Bad managers don’t. But it is also my conviction that managers generally mirror the leadership culture created by those they report to. There is, if you will, a “trickle down” effect in the style and priorities of your management.
Pharmaceutical Technology
AUGUST 16, 2022
Gilead Sciences has entered an agreement with Everest Medicines to acquire complete rights to develop and market Trodelvy (sacituzumab govitecan) in Greater China, Singapore, South Korea, Philippines, Vietnam, Thailand, Indonesia, Mongolia and Malaysia. According to the deal, Everest is entitled to receive an upfront payment of $280m from Gilead. Furthermore, Gilead will make potential payments of up to $175m to Everest on meeting certain regulatory and commercial milestones.

pharmaphorum
AUGUST 17, 2022
The clock is now ticking on the FDA’s review of GSK’s momelotinib for myelofibrosis patients with anaemia – the centrepiece of its $1.9 billion acquisition of Sierra Oncology which completed last month. The US regulator is due to make a decision on momelotinib by 16 June, 2023, on the basis of phase 3 results reported in January, said GSK.

Pharmacy Times
AUGUST 16, 2022
Insurer and pharmacy benefit manager policies used to cut the cost of drugs could be so time consuming that they could put newly diagnosed atrial fibrillation patients at risk of stroke.

Outsourcing Pharma
AUGUST 15, 2022
An expert from the clinical insight specialist shares how predictive analytics could reveal info about medication adherence, disease progression, and more.

The Guardian - Pharmaceutical Industry
AUGUST 19, 2022
Unlike jabs, nasal vaccines target the respiratory tract, the body’s first line of defence against infection People who receive a Covid booster dose in the UK next month will be among the first in the world to receive Moderna’s dual-variant vaccine , which protects against two strains of the virus. But scientists say there is a misconception that this latest vaccine is an upgrade on what has come before.
The Thyroid Pharmacist
AUGUST 19, 2022
While I have a lot of stories of people who recovered their health through removing reactive foods, adding in healing foods, and taking the appropriate supplements, I personally did not get my Hashimoto’s into remission by following those steps. Sure, I felt much better, and my thyroid antibodies significantly dropped with the help of nutrition, but eventually my health problems began creeping back in.

Pharmacy Times
AUGUST 19, 2022
Auvelity uses the first new oral mechanism of action for major depressive disorder in more than 60 years.

Pharmaceutical Technology
AUGUST 18, 2022
The US Food and Drug Administration (FDA) has granted approval for bluebird bio ’s Zynteglo (betibeglogene autotemcel, beti-cel) for the treatment of the underlying genetic cause of beta?thalassemia in adult and paediatric patients. A custom-made, one-dose gene therapy, Zynteglo is indicated for such patients who need red blood cells (RBCs) transfusions on a regular basis.

European Pharmaceutical Review
AUGUST 16, 2022
The Phase III RATIONALE 301 study evaluated the efficacy and safety of tislelizumab versus sorafenib as a first-line systemic treatment in participants with unresectable hepatocellular carcinoma. . Hepatocellular carcinoma, or HCC, is the sixth most common type of cancer worldwide. HCC accounted for more approximately 85 percent of the 900,000 new liver cancer cases in 2020, according to the Globocan 2020 database. .

The FDA Law Blog
AUGUST 16, 2022
By Douglas B. Farquhar — Those of us who work frequently on FDA inspections of drug and medical device manufacturing facilities have noticed an uptick in regular inspections after a dramatic falloff during the first two years of COVID. That impression was corroborated this week at the GMP by the Sea conference when Douglas Throckmorton, Deputy Director for Regulatory Programs at FDA’s Center for Drug Evaluation and Research, stated that domestic FDA inspections of facilities have been performed
Let's personalize your content