First Severe Case of Bird Flu Detected in Louisiana
Drug Topics
DECEMBER 20, 2024
Recent upticks of the bird flu virus in both dairy cattle and humans have also led to a state of emergency in California.

Drug Topics
DECEMBER 20, 2024
Recent upticks of the bird flu virus in both dairy cattle and humans have also led to a state of emergency in California.

Fierce Healthcare
DECEMBER 18, 2024
It still needs to pass, but an end-of-year package for healthcare is looking probable. The legislation includes PBM reform, telehealth extensions and more.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
The Checkup by Singlecare
DECEMBER 20, 2024
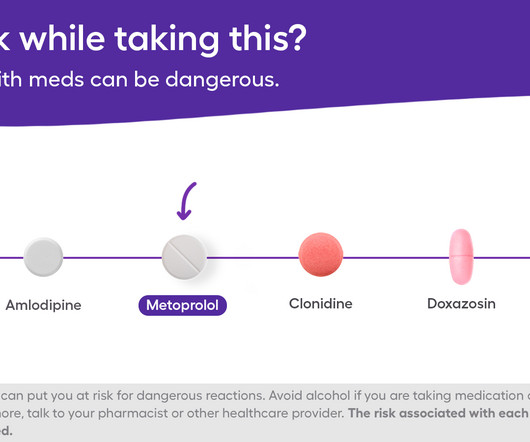
Mixing alcohol with metoprolol can come with various side effects, some of which can be serious. Many people take metoprolol, either as metoprolol tartrate or metoprolol succinate , to help manage high blood pressure and heart conditions. If youre considering drinking alcohol while taking this prescription drug, you should know the possible risks. For some people who know how they react to the medication and alcohol, drinking in moderation may be doable.

IDStewardship
DECEMBER 15, 2024
In this posta list of peculiar journal articles are highlighted, which may serve as conversationstarters from the medical literature. Authored by: Timothy P. Gauthier, Pharm.D., BCPS, BCIDP Social events like holiday parties or kids birthday parties can be unpredictable. Sometimes you know everyone and it is a lot of fun. Sometimes you do not know many people and you are stuck trying to make conversation with new acquaintances.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Topics
DECEMBER 20, 2024
With pressure from the House GOP, expected PBM reform was not included in Congress end-of-year spending package.

Fierce Pharma
DECEMBER 17, 2024
Johnson & Johnson has received coal in its stocking from the FDA as manufacturing issues have tripped up the companys attempt to gain approval of its subcutaneous version of lung cancer drug R | The FDA has sent complete response letters to Johnson & Johnson and AstraZeneca, rejecting J&J's application for subcutaneous Rybrevant and AZ's bid for a full approval of Andexxa.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

The Checkup by Singlecare
DECEMBER 19, 2024
Tens of millions of people start their day with coffee without a second thoughtmany even find it difficult to have any thoughts at all before their first cup. But if youre taking prescription drugs on a regular basis, such as Linzess (the brand name for linaclotide), you may be wondering if you need to rethink your morning joe. Linzess is an oral medication that is approved by the Food and Drug Administration for the treatment of irritable bowel syndrome with constipation (IBS-C) as well as chro

Drug Topics
DECEMBER 18, 2024
The American Pharmacists Association announced the inclusion of pharmacist-backed PBM reform in Congress end of the year spending package.

STAT
DECEMBER 20, 2024
Novo Nordisk’s next-generation obesity candidate led patients to lose a substantial amount of weight in a pivotal study but fell short of expectations, results that cast into doubt the future competitiveness of the company in the booming weight loss market. The treatment, called CagriSema, led patients to lose 20% of their weight at 68 weeks in a late-stage study when looking at all participants, including those who dropped out, Novo said Friday.

Pharmacy Times
DECEMBER 17, 2024
The investigators also find that age of initiation and frequent use were not associated with a greater age-related cognitive decline.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

The Checkup by Singlecare
DECEMBER 19, 2024
Originally approved to help people with Type 2 diabetes, the class of medications known as GLP-1 agonists and dual GIP/GLP-1s quickly acquired a reputation for helping people shed stubborn pounds. As a result, the use of medications like Ozempic , Wegovy , Zepbound , Rybelsus , and Mounjaro has skyrocketed, as people turned to these medications to help them lose weightcausing drug shortages and creating a market for compounded versions of these brand-name drugs.

Drug Topics
DECEMBER 17, 2024
Treatment of comorbidities may help patients overcome resistance or refractoriness to migraine therapies.
STAT
DECEMBER 19, 2024
Most of the formularies run by some of the largest health plans in the U.S. generally provide “fair access” to 11 treatments for several serious diseases, although transparent coverage information is often lacking for some medicines, a new analysis has found. Almost uniformly, the 11 formularies made the drugs available fairly when judged on three criteria: eligibility based on clinical data, restrictions placed on prescribers, and step therapy, which requires patients to try other
Pharmacy Times
DECEMBER 20, 2024
Vanzacaftor/tezacaftor/deutivacaftor (Alyftek) also received approval for the treatment of cystic fibrosis in people 6 years and older who have at least 1 F508del mutation.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Healthcare
DECEMBER 16, 2024
Pharmacy benefit manager reform is included in a larger-than-anticipated healthcare package, but the PBM lobby is fighting the legislation at the eleventh hour. | With a deal closer than ever, the PBM industry is hoping to sow doubt over provisions in an end-of-year healthcare package.
Drug Topics
DECEMBER 18, 2024
MannKind announced results from the INHALE-1 study that examined Afrezza in children and adolescents with diabetes.

pharmaphorum
DECEMBER 19, 2024
At its third attempt, Mesoblast has secured FDA approval for the first-ever mesenchymal stem cell (MSC) therapy, Ryoncil for acute GvHD in children.

Pharmacy Times
DECEMBER 18, 2024
The researchers suggest that an imbalance between pro-inflammatory and resolving lipid mediators may drive tumor growth.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Fierce Pharma
DECEMBER 16, 2024
Another checkpoint inhibitor has entered the fray, as Checkpoint Therapeutics has passed the FDAs checkpoint with its PD-L1 inhibitor cosibelimab. | Another checkpoint inhibitor has entered the fray, as Checkpoint Therapeutics has passed the FDAs checkpoint with its PD-L1 inhibitor cosibelimab.

Drug Topics
DECEMBER 20, 2024
Zepbound from Eli Lilly is the first and only prescription medicine approved by the FDA for adults with obstructive sleep apnea and obesity.
Fierce Healthcare
DECEMBER 18, 2024
Health AI company Suki rolled out two new features to its Suki AI Assistant powered by Google Cloud. | Suki AI released new features Thursday that brings its technology closer to a general AI assistant for clinicians from just an ambient AI scribing tool. The company touts that it is the first to release a patient summarization and search feature.
STAT
DECEMBER 19, 2024
The Food and Drug Administration on Thursday confirmed that a shortage of Eli Lilly’s obesity drug tirzepatide has been resolved, a move that will soon put a stop to companies making cheaper copies of the injection. The agency said it would give these compounders a grace period of 60 to 90 days before enforcing rules that would put a halt to their work, in an effort to avoid disruption for patients.
Pharmacy Times
DECEMBER 16, 2024
The approval builds on previous indications for tapinarof, an aryl hydrocarbon receptor agonist, for the topical treatment of atopic dermatitis.

Drug Topics
DECEMBER 19, 2024
The lawsuit alleges that the company knowingly violated both the Controlled Substances Act and the False Claims Act.

Fierce Healthcare
DECEMBER 18, 2024
AppliedVR continues to build out clinical evidence to support the use of immersive therapeutics to address chronic pain. | The company's flagship virtual reality device for chronic lower back pain delivered meaningful reductions in pain intensity for patients, particularly for high-impact chronic pain patients, according to a recent clinical study.

pharmaphorum
DECEMBER 18, 2024
The FDA has started a review of MSD's RSV antibody clesrovimab, a potential rival to Beyfortus, with a verdict due in the middle of next year

Pharmacy Times
DECEMBER 18, 2024
Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.
Drug Topics
DECEMBER 17, 2024
Student pharmacists who wore a continuous glucose monitor had a higher average counseling score during an encounter with a patient and a higher overall confidence score.

Fierce Healthcare
DECEMBER 16, 2024
Medicare Advantage has steadily grown over the past decade, and now encompasses more than half of all seniors eligible for Medicare. | Medicare Advantage has steadily grown over the past decade, and now encompasses more than half of all seniors eligible for Medicare. However, over the past year, the industry has faced significant headwinds that have stymied some of their expansion plans.

STAT
DECEMBER 14, 2024
Novo Holdings, the parent company of Novo Nordisk, can proceed with its planned $16.5 billion acquisition of Catalent, a leading contract drug manufacturer, after U.S. regulators declined to challenge the deal following months of scrutiny. The companies expect to close the transaction in the next several days, after a deadline passed for the U.S. Federal Trade Commission to raise objections and other regulatory conditions were fulfilled, according to statements issued by Novo Nordisk and Catalen
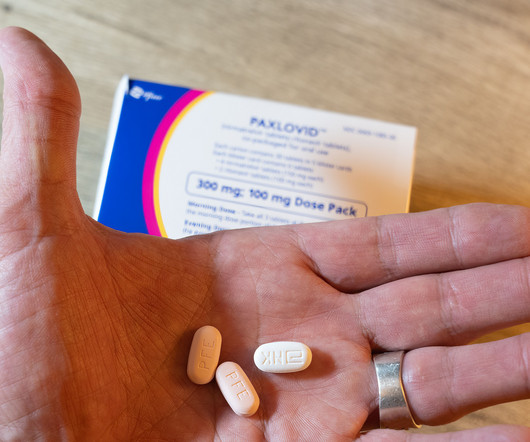
Pharmacy Times
DECEMBER 17, 2024
The conversation of VLR has sparked a discussion as to whether or not other COVID-19 treatment agents are associated with a surge in symptoms and if so, how those agents compare with the rebound effect of nirmatrelvir/ritonavir.

Drug Topics
DECEMBER 17, 2024
A conversation with Chelsee Jensen, PharmD, BCPS, senior pharmacy specialist at the Mayo Clinic.

Fierce Healthcare
DECEMBER 17, 2024
The Centers for Medicare & Medicaid Services (CMS) is discontinuing the Medicare Advantage (MA) Value-Based Insurance Design model at the end of 2025. | The Value-Based Insurance Design model for Medicare Advantage plans will be terminated at the end of next year.
Let's personalize your content