Cardiology: Most Read Stories From 2024
Drug Topics
DECEMBER 26, 2024
Check out this list of our top 5 most read cardiology stories from the year 2024.

Drug Topics
DECEMBER 26, 2024
Check out this list of our top 5 most read cardiology stories from the year 2024.
The Checkup by Singlecare
DECEMBER 26, 2024
2024 has been a busy year for new drugs. Over 40 new medications have been approved by the FDA, giving patients more options for treating various health problems. These new drugs cover a wide range of conditions, from urinary tract infections to rare genetic disorders. Notable new drugs approved in 2024 By staying up-to-date on the latest drug approvals, pharmacists can give new advice when working with patients and other healthcare providers.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmacy Times
DECEMBER 26, 2024
High levels of the Oscillibacter genus of bacteria were associated with reduced cholesterol.

Pharmaceutical Technology
DECEMBER 26, 2024
A $4.75m grant will support the development of EIS-12656, which is being studied alone and in combination with PARP inhibitors.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Pharmacy Times
DECEMBER 26, 2024
Here is an updated overview of the role of BCMA-directed therapies following the 2024 ASCO Annual Meeting and EHA Congress.

Compounding Pharmacy of America
DECEMBER 26, 2024
Discover how compounding pharmacies provide custom solutions for seniors, improving medication adherence, reducing side effects, and enhancing quality of life. The post How Compounding Pharmacies Help Seniors Easily Manage Prescriptions appeared first on Compounding Pharmacy of America.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Compounding Pharmacy of America
DECEMBER 26, 2024
Learn how dental compounding services provide personalized medication for oral health. Explore solutions for pain relief, dry sockets, and other dental needs. The post A Guide to Dental Compounding Services appeared first on Compounding Pharmacy of America.

PharmExec
DECEMBER 26, 2024
OncLive serves as the connection to oncology, including groundbreaking cancer news and interviews with top oncologists in multimedia formats.

Pharmaceutical Commerce
DECEMBER 26, 2024
The top five episodes of our podcast in the year 2024, highlighted.

PharmExec
DECEMBER 26, 2024
OncLive serves as the connection to oncology, including groundbreaking cancer news and interviews with top oncologists in multimedia formats.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharmaceutical Technology
DECEMBER 26, 2024
AstraZeneca and Daiichi Sankyo have withdrawn marketing authorisation application in the EU voluntarily, intended for datopotamab deruxtecan.

PharmExec
DECEMBER 26, 2024
In this part of his Pharmaceutical Executive video interview, Dr. Lawrence B. Werlin, MD, FACOG of HRC Fertility (@md.lawrence.werlin on TikTok), discusses the biggest misconceptions about fertility that he sees circulating on social media and work he does to correct and prevent them.

Therapeutics Education Collaboration
DECEMBER 26, 2024
In episode 594, Mike and James invite Samantha Moe back to talk about the use of the RSV vaccine in pregnancy. The only way to have an informed conversation around this vaccine is to know the numbers. If you listen to the whole podcast, youll get all the numbers available to date. Show notes Tools for Practice 1)Bumpin Up the Protection? RSV Vaccine in Pregnancy 2) Preventing RSV Infections in Infants 3) Preventing RSV in the elderly In episode 594, Mike and James invite Samantha Moe back to tal

Pharmaceutical Technology
DECEMBER 26, 2024
Immune globulin (human) is under clinical development by Grifols and currently in Phase II for Tachycardia (Tachyarrhythmias).

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
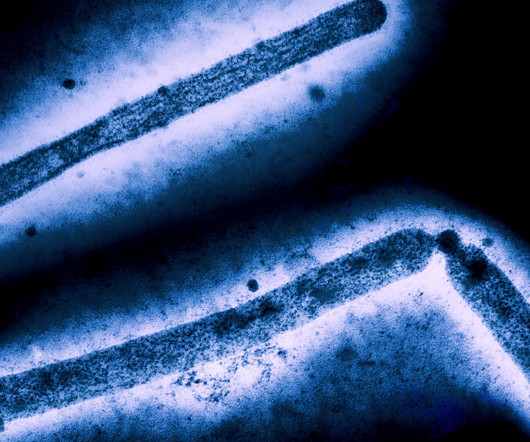
STAT
DECEMBER 26, 2024
Genetic sequences of H5N1 bird flu viruses collected from a person in Louisiana who became severely ill show signs of development of several mutations thought to affect the virus’ ability to attach to cells in the upper airways of humans, the Centers for Disease Control and Prevention reported on Thursday. One of the mutations was also seen in a virus sample taken from a teenager in British Columbia who was in critical condition in a Vancouver hospital for weeks after contracting H5N1.

Pharmaceutical Technology
DECEMBER 26, 2024
Allergen for Grass Pollen Allergy is under clinical development by Roxall Medizin and currently in Phase II for Grass Pollen Allergy.

STAT
DECEMBER 26, 2024
The global medical community encountered a highly contagious aerosolized pathogen with no known treatment when the SARS-CoV 2-virus triggered the Covid-19 global pandemic nearly five years ago. Fortunately, Covid responded to treatment with convalescent plasma while other therapies and vaccinations were under development. Today, the H5N1 avian flu virus, currently lurking in birds and cows, is perhaps only a few mutations away from a potentially similar widespread and deadly outbreak in hu

Pharmaceutical Technology
DECEMBER 26, 2024
Darigabat is under clinical development by Cerevel Therapeutics and currently in Phase II for Seizures.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

STAT
DECEMBER 26, 2024
In 2024, STAT’s reporters brought you tons of great journalism, and elsewhere [link to in-case-you-missed it] we’ve given you a compendium of the stories you may have missed on our site. We’ve also collected for you below our annual list of stories that STAT staffers loved, and wish that they had written. (Also check out the jealousy list at Bloomberg Businessweek, which had the idea first.

Pharmaceutical Technology
DECEMBER 26, 2024
Nelivaptan is under clinical development by HMNC and currently in Phase II for Major Depressive Disorder.

STAT
DECEMBER 26, 2024
Here we are, on the cusp of the midway point in a decade that has been, in global health and infectious diseases terms, a lot. The 2020s started with the most severe pandemic since the 1918 Spanish flu. Just as the worst of Covid-19 was starting to ease, the world was introduced to mpox, a cousin of smallpox that went from occasionally infecting people who had contact with infected rodents in forested parts of West and Central Africa to spreading from person to person in Europe, the Americas, an

Pharmaceutical Technology
DECEMBER 26, 2024
Gene Therapy to Target GD2 for Oncology is under clinical development by Bristol-Myers Squibb and currently in Phase I for Sarcomas.

STAT
DECEMBER 26, 2024
If the last few months are any guide, there will be no shortage of surprises and challenges for the biotech industry in the new year. Donald Trump will begin his second term, possibly bringing along controversial figures to lead health agencies. And biotech startups, which have already endured a prolonged economic downturn, are set to face further financial strain as the Federal Reserve projects higher inflation and fewer interest rate cuts next year.
Pharmaceutical Technology
DECEMBER 26, 2024
Gene Therapy to Target GD2 for Oncology is under clinical development by Bristol-Myers Squibb and currently in Phase I for Osteosarcoma.

Pharmaceutical Technology
DECEMBER 26, 2024
Tricaprilin is under clinical development by Cerecin and currently in Phase II for Infantile Spasm (West Syndrome).

Pharmaceutical Technology
DECEMBER 26, 2024
KGYY-15 is under clinical development by Op-T-Mune and currently in Phase I for Type 1 Diabetes (Juvenile Diabetes).

Pharmaceutical Technology
DECEMBER 26, 2024
Lidocaine is under clinical development by MEDRx and currently in Pre-Registration for Postherpetic Neuralgia.

Pharmaceutical Technology
DECEMBER 26, 2024
StemVacs-V is under clinical development by Res Nova Bio and currently in Phase II for Metastatic Breast Cancer.
Let's personalize your content