A Proposal for a Comprehensive Quality Overall Summary
ISPE
MARCH 29, 2023
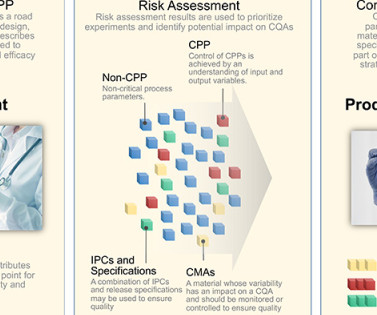
The TPP defines the indication and patient population, describes usage of the product, provides dosage and administration details, explains dosage form and strengths, and lists packaging and storage requirements. Specification criteria are often established to control CMAs. CPP 3 IPC Film coat weight gain (P.3.4)










Let's personalize your content