New Trends & Requirements from Digitalization, Annex 1, Continuous Manufacturing & High Potent Manufacturing
ISPE
MARCH 16, 2023
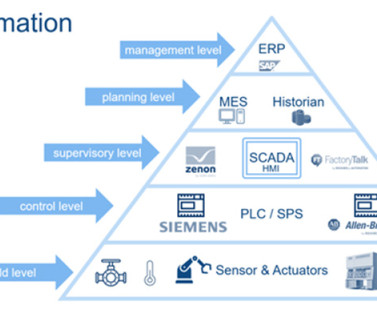
By removing the need for manual handling and reducing the risk of contamination, pharmaceutical manufacturers can produce products that meet the highest standards of quality. Automated systems offer a range of options for pharmaceutical companies. This also results in significant cost savings and increased efficiency.






















Let's personalize your content