CMC Requirements for New Drug Registration in Latin America
ISPE
MAY 9, 2023
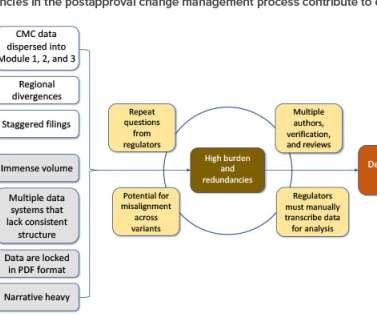
Improvements have been observed by various regulatory agencies in Latin America for the acceptance and implementation of international standards—for instance, the ICH Common Technical Document (CTD) format. Additional required information beyond ICH guidelines, or non-value-added documents (e.g., 6, and 2.3.S.7 7 are required.








Let's personalize your content