STAT+: Compounding group sues FDA for removing Lilly’s obesity drug from its shortages list
STAT
OCTOBER 8, 2024
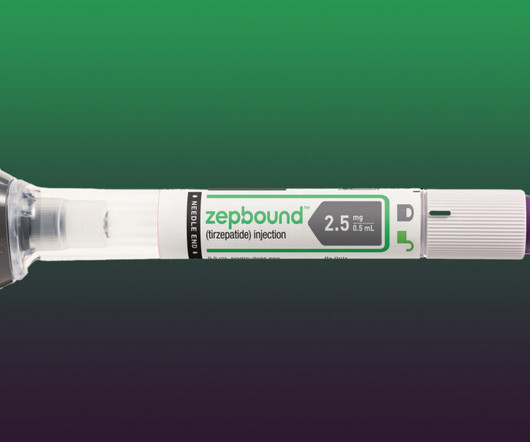
A trade group representing large compounding pharmacies has sued the U.S. Food and Drug Administration for a “reckless and arbitrary” decision to remove a widely prescribed Eli Lilly drug for combating diabetes and obesity from an official shortages list.
















































Let's personalize your content