Dengue is spreading and Sanofi is pulling the market’s only vaccine. What’s next?
PharmaVoice
OCTOBER 23, 2024
As Sanofi prepares to halt production on its dengue vaccine in 2026, locally acquired infections in the U.S. are raising the alarm.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

PharmaVoice
OCTOBER 23, 2024
As Sanofi prepares to halt production on its dengue vaccine in 2026, locally acquired infections in the U.S. are raising the alarm.
Pharmacy Times
APRIL 2, 2025
The only major change is in the included strain of influenza A/H2N2 virus, which has been updated for both the egg-based and the cell- and recombinant-based vaccines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 6, 2024
As BioNTech continues to endure a sharp decline in COVID-19 vaccine sales, the German mRNA specialist is looking ahead to the next leg of its commercial journey. With plans to have at least 10 potentially registrational trials underway by the end of 2024, BioNTech is plotting the first wave of its market debut in oncology from 2026 onward.

STAT
OCTOBER 11, 2023
A company that Pfizer blamed for problems with a clinical trial testing a Lyme vaccine claims that regulators gave its procedures a clean bill of health during a recent inspection. Care Access served as a contract research organization and had enrolled about 3,000 patients in the late-stage trial.

STAT
AUGUST 22, 2023
The Food and Drug Administration approved a Pfizer vaccine that aims to protect newborns against RSV by vaccinating pregnant people, STAT tells us. The vaccine, marketed as Abrysvo, previously won approval for adults over the age of 60. Continue to STAT+ to read the full story…

Pharmaceutical Technology
JULY 18, 2022
GlaxoSmithKline (GSK) has signed an agreement with the Government of Canada for pandemic and seasonal influenza vaccines to aid in protecting adults and children in the country. The company intends to supply both vaccines from its 230,000ft² Sainte-Foy facility in Quebec. .

Pharmaceutical Technology
NOVEMBER 24, 2022
South African bio-pharmaceutical firm Biovac and the International Vaccine Institute (IVI) in South Korea have signed a licencing and technology transfer agreement for developing and manufacturing an oral cholera vaccine (OCV).

Pharmaceutical Technology
JUNE 2, 2023
The European Commission (EC) and Pfizer/BioNTech have agreed to amend their current supply contract to deliver Covid-19 vaccines to the EU. More significantly, the revised contract secures about 70 million vaccine doses every year from 2023 to 2026 for member states, leaving little space in the market for competitors.

pharmaphorum
JUNE 15, 2021
New Science is projected to drive 81% of biopharma revenue growth and 61% of all revenues between 2021 and 2026, outpacing our previous forecast of 54% of all revenues from 2017-2022. And we expect a 69% higher average net present value (NPV) for New Science treatments versus traditional counterparts from 2022—2026.

PQA
JANUARY 12, 2024
Looking ahead, we are excited to announce dates for the 2024 Leadership Summit, as well as the 2025 and 2026 Annual Meetings. and in 2026, we'll be back in Baltimore. Save the dates for PQA's upcoming major meetings now through 2026! We know you have busy schedules and many meetings to attend throughout the year. Save the Dates!

pharmaphorum
APRIL 29, 2022
The healthy sales increase was bolstered by the timing of orders for AZ’s COVID-19 vaccine Vaxzevria , which made $1.1 “Our combined company has already successfully leveraged internal scientific synergies, and this move will act as a catalyst for even more external collaboration and innovation,” he added.

pharmaphorum
OCTOBER 28, 2021
The Association of the British Pharmaceutical Industry (ABPI) and BioIndustry Association (BIA) both said that the increase acknowledges the enormous contribution made by the life sciences sector in helping to limit the impact of the pandemic through the rapid development of diagnostics, drugs and vaccines.

The FDA Law Blog
DECEMBER 3, 2023
On November 17th, CMS issued its final guidance on the Discount Program in which it responded to public comments and provided updated guidance for the Discount Program for 2025 and 2026. covered insulin product or vaccine). Manufacturers must sign the agreement by March 1, 2024, to participate in the 2025 plan year.

Pharmaceutical Technology
SEPTEMBER 23, 2022
It will expire on 24 November 2026, and the paediatric exclusivity lasts until 24 May 2027. Additionally, Merck has signed patent litigation settlement deals with various generic firms, permitting them to offer generic versions of Januvia and Janumet to the US market in May 2026, or before under specific conditions.

European Pharmaceutical Review
AUGUST 22, 2022
They noted that while production of COVID-19 pandemic vaccines and therapeutics is currently absorbing much of the flexible capacity available in the industry, but said that as demand moderates, capacity will normalise enabling other products to be prioritised.

pharmaphorum
SEPTEMBER 30, 2022
That challenged the ‘708 patent – which expires in November 2026 – as well as one other (No. Viatris could still take its cases further, but for now Merck has retained market exclusivity for Januvia, Janumet, and Janumet XR until at least 2026. billion in the first half of 2022.

pharmaphorum
JANUARY 29, 2023
Think about recent technological breakthroughs such as mRNA vaccines and the potential for mRNA-based therapeutics, or the recent ramp up in vaccine production facilities – to name just a few examples. That’s what is relentlessly driving industry growth, as well as spurring innovation and efficiency.

European Pharmaceutical Review
SEPTEMBER 22, 2023
Aseptic packaging eliminates the necessity for preservatives and refrigeration which is especially advantageous for medications and vaccines that are sensitive to temperature changes. Research Nester highlighted statistics which acknowledged that European production of pharmaceutical aseptic packaging is expected to reach $56 billion by 2026.

pharmaphorum
FEBRUARY 7, 2022
The approval is some relief for Sanofi after a crop of pipeline disappointments last year, with Evaluate estimating the drug could make more than $500 million in sales in 2026. It also abandoned an mRNA-based vaccine for COVID-19 and suffered delays to another COVID-19 shot partnered with GlaxoSmithKline.

pharmaphorum
JANUARY 21, 2022
And as with many healthcare challenges, it is an issue that has been brought into sharp focus by COVID-19, during which we have seen huge investments in ground-breaking vaccines, quickly followed by inequities in access. There is no easy answer to the question of how to incentivise innovation without restricting access.

STAT
JULY 30, 2024
… Four pharmaceutical companies involved in the negotiations over prices for Medicare do not expect a significant impact on their businesses after seeing confidential suggested prices from the government for their drugs that will take effect in 2026 , Reuters writes.

PharmaShots
FEBRUARY 21, 2023
threshold by 2026. Under its cancer therapy products, the company develops low molecular drugs, antibody drugs, cancer peptide vaccines, and diagnostic medicines. With the growing influence of radiopharmaceutical companies in oncology, the companies hold a firm grasp on the market.

ISPE
SEPTEMBER 29, 2023
For more than 240 years, Takeda has focused on delivering transformative treatments in the therapeutic areas of gastrointestinal and inflammation, rare diseases, plasma-derived therapies, oncology, neuroscience, and vaccines. Takeda has reduced CO2 emission by 861 tons.

pharmaphorum
FEBRUARY 16, 2022
The Life Sciences Vision also identified seven therapeutic areas that are priorities for the government: treatments for neurodegenerative disorders, cancer immunotherapies, novel vaccines, cardiometabolic therapies, treatments for respiratory disorders, therapies for multi-system ageing and treatments for mental illness.

pharmaphorum
JULY 1, 2021
Along with the appointment of new non-executive directors, Elliott would like to see a moratorium on profit and revenue-related bonuses unless management meets its 2026 targets of 5% profit growth and 10% turnover growth and raising the margin target from 30% to 32%.

Pharmaceutical Technology
MAY 25, 2023
Analysis estimates that the oncolytic virus market will reach $1 billion by 2026. Transgene will be presenting new data at ASCO 2023 for its therapeutic cancer vaccines TG4001, in Phase II study in patients with HPV16-positive anogenital tumours ( NCT03260023 ), and TG4050, Phase I study in head and neck cancers ( NCT04183166 ).

pharmaphorum
APRIL 21, 2022
Overall spending on medicines rose 12% in 2021 to $407 billion, driven by a $29 billion spend on COVID-19 vaccines and medicines. Prescription drug use in the US soared last year, as might be expected during a pandemic, but is expected to fall back sharply in 2023, according to IQVIA’s latest report on the sector.

European Pharmaceutical Review
JULY 6, 2023
Further trials will be launching although most patients are expected to be enrolled from 2026 onwards. The Cancer Vaccine Launch Pad (CVLP), a new partnership led by NHS England, will create a database of eligible NHS cancer patients who will be offered the choice to take part in personalised cancer vaccine trials.
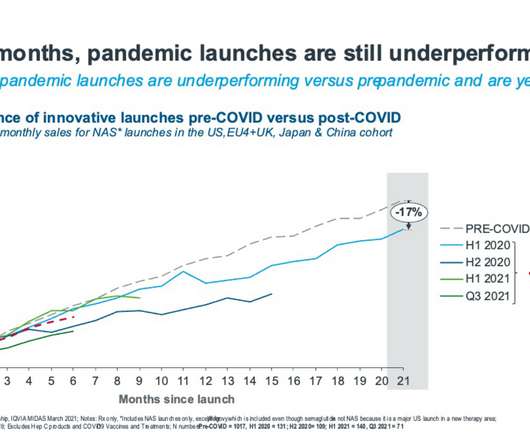
pharmaphorum
JUNE 29, 2022
COVID vaccines and treatments have created a substantial market over and above the existing Rx market- IQVIA estimates that the cumulative value of COVID vaccines could be between $185 and 295bn to 2026. The prescription medicine market has recovered from the wild swings of the early pandemic with renewed growth.

Fierce Pharma
OCTOBER 9, 2023
GSK has forged a partnership with vaccine powerhouse Chongqing | GSK has forged a partnership with vaccine powerhouse Chongqing Zhifei Biological Products to distribute Shingrix in China. billion) for exclusive rights to distribute the shingles shot in China from 2024 through 2026. Zhifei will pay £2.5 billion ($3.05

Pharmaceutical Technology
JULY 28, 2022
Tuas Biomedical Park New Vaccine Production Facility – $467m. The project involves the construction of a new vaccine production facilities at Tuas Biomedical Park in Singapore. Construction work started in Q2 2022 and is expected to be completed in Q1 2026. Yeosu Mononitrobenzene Manufacturing Plant – $375m.

Pharmaceutical Technology
JULY 28, 2022
Tuas Biomedical Park New Vaccine Production Facility – $467m. The project involves the construction of a new vaccine production facilities at Tuas Biomedical Park in Singapore. Construction work started in Q2 2022 and is expected to be completed in Q1 2026. Yeosu Mononitrobenzene Manufacturing Plant – $375m.

STAT
DECEMBER 10, 2024
Democrats floated the idea of extending the subsidies through 2026 in a year-end deal, and paying for it with a budgetary maneuver that would theoretically extend budget cuts that haven’t actually been implemented, as my colleague Rachel Cohrs Zhang reported. million people would lose coverage annually without the tax credits.

The FDA Law Blog
AUGUST 14, 2022
The number will begin in 2026 with 10 drug covered under Medicare Part D and will increase annually to 20 Part B and 20 Part D drugs by 2029 and thereafter, with the selected drugs accumulating from year to year. The same will be required under state Medicaid and CHIP programs by October 1, 2023.

pharmaphorum
SEPTEMBER 8, 2022
On the strength of phase 2 data, the FDA has awarded coveted breakthrough status to Pfizer’s vaccine against Group B Streptococcus (GBS), given to expectant mothers to prevent invasive infections in newborns and young infants. The post FDA gives Pfizer’s Group B strep vaccine breakthrough status appeared first on.

pharmaphorum
JANUARY 6, 2022
IQVIA Institute models global cumulative spend on COVID-19 vaccines to be a base case of $251bn to 2026 , with spend on COVID-19 treatments, including the new oral agents and treatments of post-viral syndromes, likely to add significant incremental spend. Medicine budget constraint, structural fund largesse.

The Checkup by Singlecare
JUNE 6, 2024
Farxiga was selected as one of the first 10 drugs for negotiated prices and should be available at lower prices starting in 2026. The good news is that all Medicare Part D drug plans offer coverage for at least two drugs within commonly prescribed drug categories.

Viseven
SEPTEMBER 18, 2024
The rest is history: vaccines were delivered worldwide in record time, saving millions of lives. By the end of 2026, the company will be able to analyze two million genomes. This quick reporting helped the CDC gather crucial data on the vaccine’s effectiveness, contributing to its further improvements.

The Checkup by Singlecare
JUNE 6, 2024
That means a new, negotiated price for Jardiance will be effective in 2026. And according to Medicare, negotiated prices will benefit Medicare enrollees in several ways: More vaccines covered – People with Medicare can now receive several vaccines— shingles , whooping cough , and more—at zero cost.

pharmaphorum
NOVEMBER 6, 2022
The new drug will reach the market ahead of RSV vaccines that are designed to protect newborns in the months after birth by vaccinating expectant mothers, who pass on antibodies against the virus in the womb. AZ and Sanofi say they hope to launch the antibody next autumn, in time for the 2023/24 RSV season.

The FDA Law Blog
FEBRUARY 4, 2025
With the current lapse in authorization, FDA may only award RPD PRVs for applications that have received an RPD designation before December 20, 2024, andunless reauthorized before thenmay not award any RPD PRVs after September 30, 2026. Below, we discuss some of its downstream impacts on the PRV landscape.

pharmaphorum
OCTOBER 21, 2020
Merck & Co has built more momentum behind its attempt to depose Pfizer’s blockbuster pneumococcal conjugate vaccine Prevnar 13, with two new phase 3 trials backing its rival shot V114. It reiterated its plans to file for approval of the vaccine before the end of the year in the US.

BuzzRx
AUGUST 30, 2022
The Inflation Reduction Act will allow the government to negotiate lower prescription drug costs for some of the highest-priced, covered drugs between 2026 and 2029 and beyond. million who received the shingles vaccine. These are mainly drugs for which generic or biosimilar competitors are not available.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content