Challenges of analytical method validation for ATMPs
pharmaphorum
JUNE 22, 2023
Challenges of analytical method validation for ATMPs Mike.Hammerton Thu, 22/06/2023 - 08:00 Bookmark this

pharmaphorum
JUNE 22, 2023
Challenges of analytical method validation for ATMPs Mike.Hammerton Thu, 22/06/2023 - 08:00 Bookmark this

European Pharmaceutical Review
MARCH 6, 2024
Additionally, the new laboratory offers Ariceum the ability to carry out on-site process, method, validation and formulation development as well as preliminary stability analysis of its radiolabeled compounds. Internet] European Pharmaceutical Review, Issue 3 2023. 1 References Jaafar-Thiel. cited 2024Mar].
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

ISPE
JUNE 2, 2023
reduce non-value-added tests) Contract lab management Reference standards & critical reagent management Rapid micro testing Training (e.g.,

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. This document is revised from a version published in October 2023.

ISPE
MAY 10, 2023
CoP Leader Profiles: Roujian “RJ” Zhang Trudy Patterson Wed, 05/10/2023 - 11:37 InTouch May / June 2023 CoP Leader Profiles: Roujian “RJ” Zhang Marcy Sanford 1 May 2023 Roujian “RJ” Zhang is Chair of ISPE’s new Quality Control (QC)/Analytical Community of Practice (CoP) Steering Committee.
ISPE
JANUARY 11, 2023
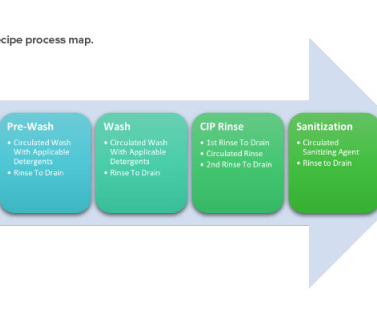
Life-Cycle Approach to Cleaning Topical Drug Products Trudy Patterson Wed, 01/11/2023 - 10:17 Technical January / February 2023 Life-Cycle Approach to Cleaning Topical Drug Products Dijana Hadziselimovic Kamini I. The cleaning validation life-cycle approach consists of three stages: design, qualification, and continued verification.

European Pharmaceutical Review
JANUARY 26, 2023
The European Medicines Agency (EMA) has published an updated Q&A document regarding ICH M10 ‘Bioanalytical Method Validation and Study Sample Analysis’. The ICH M10 guideline provides recommendations on the validation of bioanalytical assays for chemical and biological drugs and their metabolites in biological matrices.
Let's personalize your content