Pharmaceutical microbiology: key developments 2022
European Pharmaceutical Review
JANUARY 16, 2023
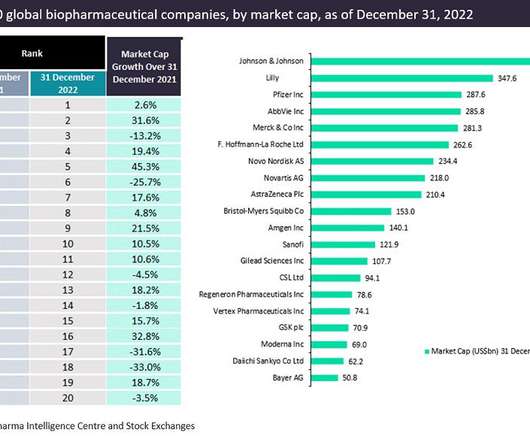
This Q&A covers the key developments in pharmaceutical microbiology in 2022, featuring insight from pharmaceutical microbiology experts: Dr Tim Sandle, Head of Microbiology, Bio Products Laboratory Limited. Regulation, particularly Annex 1, was identified as a key focus for 2022.














































Let's personalize your content