Industry Voices—Not all automation is created equally for clinical documentation improvement
Fierce Healthcare
SEPTEMBER 15, 2021
Industry Voices—Not all automation is created equally for clinical documentation improvement. Wed, 09/15/2021 - 15:26.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Fierce Healthcare
SEPTEMBER 15, 2021
Industry Voices—Not all automation is created equally for clinical documentation improvement. Wed, 09/15/2021 - 15:26.

pharmaphorum
NOVEMBER 13, 2020
Join us at our 2nd Clinical Document World Virtual event on January 19-21, 2021, alongside your TMF, clinical quality, document management, and clinical professionals to explore a strategic clinical process and ensure a complete Document Management Process. Focus on ensuring high quality documentation.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
MARCH 20, 2024
Brackbill, who joined J&J in the finance department in 1999, joined this group in 2021. The work includes establishing distribution channels, pricing, strategic account management, and contracting across the portfolio of medicines, according to the lawsuit. Continue to STAT+ to read the full story…

pharmaphorum
DECEMBER 22, 2021
As part of our 2021 in Review series, we look back over some of these developments. The document also states the importance of real-world data in understanding how currently available pharmaceutical products work in different populations. Education and empowerment allow for equal access to care. Looking ahead.

pharmaphorum
JANUARY 12, 2021
2020 was a big year for market access initiatives in the UK, many of which are only just starting, and their impact will come through in 2021 and beyond. It’ll also reveal just what the Target Development Profile (TDP) living document will cover and how it can evolve over time. That makes it a target for June 2021.

pharmaphorum
JUNE 22, 2021
The UK’s Department of Health and Social Care has published a document setting out its strategy on handling patient data – and defending its plan to transfer millions of GP records into a centralised database. — Matt Hancock (@MattHancock) June 22, 2021. — medConfidential (@medConfidential) June 22, 2021.

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. After reviewing comments, FDA will revise and move questions and answers to the final document, as it deems appropriate. By Sophia R. Gaulkin — Last week, the U.S.

STAT
JULY 10, 2024
This document is where the government has in the past rolled out changes to the so-called hospital price transparency rule, but the Biden administration did not address the issue in this edition. Since 2021, hospitals have been required to post the prices they have negotiated with all health insurance companies, as well as their cash prices.

STAT
OCTOBER 4, 2023
At issue is a trial that Amgen is relying upon to win final approval for Lumakras, which the FDA approved on a conditional basis in 2021. But ahead of an advisory panel meeting on Thursday, the agency released documents showing its staff found “potential systemic bias” in the trial.

IDStewardship
OCTOBER 28, 2022
Develop a manual or guidance document outlining the goals of the account. This type of document is especially helpful for account management transitions in resident managed accounts. Multiple pharmacy organizations have guidance or best practice documents pertaining to social media use. UKPharmRes. BIDMCPharmRes. MayoPharmRes.

European Pharmaceutical Review
JANUARY 17, 2023
The figures confirmed that in 2022, the UK remained a global leader in clinical research, with total ongoing trials increasing from 168 in 2021 to 178 in 2022. The number of Phase II/III trials increased from three trials in 2021 to seven trials in 2022. Over 80 percent, increasing from 131 trials in 2021 to 145 in 2022.

pharmaphorum
JULY 6, 2021
Oxford Nanopore is also building up to an initial public offering (IPO) later this year that could reinforce 2021’s position as the best-ever year for UK financing, well ahead of last year’s total tally of £2.81 Source: BIA, Clarivate.

pharmaphorum
SEPTEMBER 2, 2020
The guidance published on the Medicines and Healthcare products Regulatory Agency (MHRA) website is broadly similar to arrangements laid out in a Brexit “no deal” document published last year. Companies face significant changes in how the complex environment for medicines regulations will operate in 2021. “If

STAT
DECEMBER 29, 2022
The downfall of Aduhelm, the first new treatment for Alzheimer’s disease in two decades, is largely the story of a drug company choosing to maximize its potential profits at the expense of patients and taxpayers, according to a congressional investigation that cites thousands of pages of internal Biogen documents.

Med Ed 101
MARCH 10, 2021
This document […]. The post Heart Failure Guideline Updates in 2021 appeared first on Med Ed 101. Prior to the full guideline release, the organization recently printed their updated Expert Consensus Decision Pathway for treatment of patients with heart failure with reduced ejection fraction (HFrEF).

pharmaphorum
OCTOBER 18, 2021
2 And, as of September 2021, there are 22 COVID-19 vaccines in use globally 3 , and over 2.3 And advances in digitalisation could facilitate a move from document-based submissions to data-based submissions, through a cloud platform like the one being developed by Accumulus Synergy. Last accessed: October 2021. Perspect Clin Res.

pharmaphorum
DECEMBER 4, 2020
In a separate announcement, rival vaccine firm Moderna said it expected to have between 100 and 125 million doses available globally in the first quarter of 2021. The company said it is still on course to manufacture between 500 million and up to a billion doses of the vaccine globally in 2021.

ISPE
OCTOBER 28, 2022
The 2021 publication of the ISPE Good Practice Guide: Good Engineering Practice, 2nd ed., (GEP Application of engineering change management (ECM), engineering document management, and engineering issue management throughout the C&Q process can significantly reduce time, cost, effort, and risk. through system acceptance and release).

The FDA Law Blog
NOVEMBER 6, 2023
A phone call to FDA requested information about the number of Remote Interactive Evaluations (RIEs) that FDA has performed at drug manufacturing facilities since it announced in April 2021 that it would start using them as an alternative to on-site inspections. We should explain what RIEs are. Comments on the guidance are invited.

European Pharmaceutical Review
NOVEMBER 21, 2022
Study data from Elsevier’s abstract and citation database Scopus, identified that 2,450 papers were published in 2021 on the pathogen WHO identified as most critical, Acinetobacter baumannii (carbapenem-resistant). Enterococcus faecium : 1,246 in 2021 versus 730 in 2017. Neisseria gonorrhoeae : 731 in 2021 versus 478 in 2017.

European Pharmaceutical Review
MARCH 13, 2024
The data integrity violations API deviations in the microbiology lab The first deviation – failure to follow and document laboratory controls at the time of performance, and failure to document and explain any departures from laboratory procedures – relates to the lack of data integrity within the microbiology laboratory.

IDStewardship
MARCH 12, 2023
Memorizing 45+ page document is certainly not a reasonable expectation, but one can certainly walk away with an awareness of general concepts and themes which are relevant. Article 5: A Baker’s Dozen of Top Antimicrobial Stewardship Intervention Publications for Hospitalized Patients in 2021 Find it here.

Pharmaceutical Technology
JULY 19, 2022
Mentions of cloud computing within the filings of companies in the pharmaceutical industry rose 65% between the final quarter of 2021 and the first quarter of 2022. Of the 10 biggest employers in the pharmaceutical industry, IQVIA was the company which referred to cloud computing the most between April 2021 and March 2022.
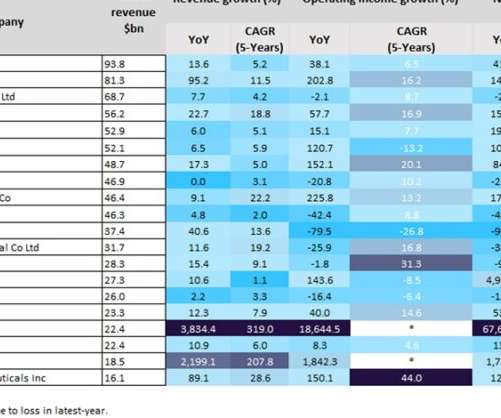
Pharmaceutical Technology
SEPTEMBER 15, 2022
The top 13 players reported more than 10% revenue growth, with BioNTech (3,834.4%), Moderna (2,199.1%), Pfizer (95.2%) and Regeneron Pharmaceuticals (89.1%) reporting a more than 80% year-on-year (YoY) revenue growth from 2020 to 2021, according to GlobalData’s Pharma Intelligence Centre Companies Database. AbbVie reported a 22.7%

pharmaphorum
FEBRUARY 10, 2021
Pharmaceutical companies will need new standard operating procedures (SOP) to cover activities such as collaborative working, and new documentation to reflect changes in language. But while amending processes and documentation to suit the 2021 code won’t be easy, the task doesn’t have to be as overwhelming as it first appears, she said.

pharmaphorum
MARCH 7, 2022
Last November, it was confirmed that both NHS Digital and NHSX are to be folded into NHS England’s new Transformation Directorate, set up earlier in 2021 with the aim of bringing together digital and operational improvement teams within the NHS. — medConfidential (@medConfidential) November 22, 2021.

Med Ed 101
JUNE 9, 2021
The post Preparing For The BCMTMS Exam in 2021-2022 appeared first on Med Ed 101. I’m happy with the testimonials that we have received that make me believe we provide the best resources on the market that […].

ISPE
MARCH 23, 2023
A True Copy is an exact copy of original documentation that preserves the same content, meaning and attributes of the original. It is an electronic copy maintained in an electronic document management system. This negates the requirement for storage of paper evidence and correct GMP document management of these hardcopy evidences.

The FDA Law Blog
DECEMBER 4, 2023
Prescribing Red Flags The government alleged that from at least 2017 to April 2021 Defendants knowingly filled controlled substance prescriptions “that raised obvious ’red flags’ of potential abuse or diversion.” If the pharmacist can resolve it, they must make a record of the resolution. Complaint ¶ 55.

European Pharmaceutical Review
SEPTEMBER 2, 2022
The sampling strategy must be supported by sound and properly cited sources whose conclusions must be presented as supportive elements in the study documents. The following four elements of sampling methodology were found to be under-documented in RMM effectiveness studies: Supporting documentation for country/region selection.

Druggist
MAY 6, 2021
The official guide on type-2 diabetes management: document produced by NICE for healthcare professionals in the UK, which recommends treatment for type-2 diabetes. . By far, the most widely used sulfonylurea in the UK is gliclazide (OpenPrescribing.net, 2021). Available at: [link] Accessed on 04/05/2021. eMC (2021).

The FDA Law Blog
SEPTEMBER 10, 2023
Tobolowsky — CDER, CBER, and the Oncology Center of Excellence recently published a final guidance document titled “ Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug and Biological Products ” as another part of its real-world evidence (“RWE”) Program.

Pharmaceutical Technology
JULY 15, 2022
The documents state that a partnership with a CMO would be cheaper than the state directly manufacturing insulin. California’s budget documents give few details about the tender process, but state that CalRx will spend $50 million to partner with a contract manufacturer to develop biosimilar insulin products in vial and pen form.

pharmaphorum
JANUARY 28, 2022
But never mind, 2021 was a blockbuster even compared to 2020 or 2019, with many sources saying there was twice as much, or nearly twice as much money raised this past year compared with the year before. In this article I’m looking at four 2021 healthcare funding reports: CB Insights, StartUp Health, Rock Health, and Silicon Valley Bank.

pharmaphorum
DECEMBER 16, 2020
The document has been published just after the US started the rollout of Pfizer and BioNTech’s vaccine after it got an emergency green light last week, and ahead of an expert panel due to consider Moderna’s shot on Thursday. The post FDA backs Moderna COVID-19 shot ahead of emergency use vote appeared first on.

pharmaphorum
JUNE 3, 2021
This article appears in our digital magazine Deep Dive: Market Access 2021. And anyone interested in a game of policy bingo will be able to mark off all the important policy documents and every agency too. Leela Barham takes stock. Read below for a preview: NICE has never stood still since it started its work in 1999.

pharmaphorum
MAY 16, 2022
In 2020, USAID launched two policy documents to guide how the agency invests in digital infrastructure as part of its development and humanitarian assistance programmes: Digital Strategy and Vision for Action in Digital Health (aka Digital Health Vision). “Economists in 2021 estimated the pandemic cost the world over $11 trillion.

European Pharmaceutical Review
OCTOBER 25, 2023
The document by the Medicines and Healthcare products Regulatory Agency (MHRA), US Food and Drug Administration (FDA) and Health Canada, contains key guidance on predetermined change control plans (PCCPs) for MLMDs.

The FDA Law Blog
OCTOBER 18, 2022
Amongst CDRH’s highest prioritized device guidance documents are final versions of the transition plans for medical devices that fall within enforcement policies or that were issued emergency use authorizations (EUAs) during Covid-19 public health emergency (PHE). They are also open to information to include in these guidance documents.

Pharma Marketing Network
APRIL 30, 2021
At this year’s Reuters Pharma Clinical 2021, I joined over 500 global leaders in clinical research as they discussed many of these issues under the theme, “rebuild clinical trials in the image of patient need.”. How do we increase the diversity of clinical trials?
pharmaphorum
NOVEMBER 16, 2021
NICE notes in its final appraisal document (FAD) for the drug that many patients with this form of epilepsy may have to try a range of medicines, both alone and in combination, in order to control seizures. An opinion from the Scottish Medicine Consortium (SMC) on the use of the drug by NHS Scotland is expected to be published early in 2022.

pharmaphorum
NOVEMBER 20, 2020
billion doses by the end of 2021. It is one of 11 vaccines that are in phase 3 development, including contenders from Moderna, Novavax and AstraZeneca, according to the World Health Organization’s regularly updated tracker document. Feature image courtesy of NIAID/Rocky Mountain Laboratories.

pharmaphorum
JUNE 2, 2021
The document – which is available in digital or paper form – will be “free of charge, secure and accessible to all,” according to the European Commission, and may be scanned at the point of departure or arrival within the EU. — Ursula von der Leyen (@vonderleyen) May 31, 2021.

pharmaphorum
OCTOBER 21, 2021
It can be given to patients who have the most debilitating form AHP, with four or more severe attacks a year, according to a final appraisal document from NICE, which reverses an earlier position that the drug should not be made available by the NHS. — British Porphyria Association (@BPA_Porphyria) October 21, 2021.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content