Vaccine Hesitancy Trends from 2019 to 2022 Evaluated
Drug Topics
OCTOBER 21, 2022
As vaccine hesitancy grows, fewer children are receiving routine childhood vaccines.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Drug Topics
OCTOBER 21, 2022
As vaccine hesitancy grows, fewer children are receiving routine childhood vaccines.
Drug Topics
JANUARY 3, 2025
Researchers aimed to identify the barriers impeding HPV vaccine uptake since 2019 among US mid-adults aged 27 to 45.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmacy Times
JUNE 27, 2022
The FDA approved the first vaccine for dengue disease in May 2019.

STAT
JANUARY 12, 2023
kindergartners who’ve received standard childhood vaccines took a small but notable dip into the 2021-2022 school year, health officials said Thursday, amid disruptions related to Covid-19 and fears that anti-vaccine sentiment stirred up by the pandemic could be spreading to other shots. The percentage of U.S.

Pharmacy Times
NOVEMBER 16, 2022
In a 2019 survey, middle-aged participants were considerably more apprehensive about getting vaccinated than younger groups, which was not the case in the 2022 survey.

pharmaphorum
JUNE 21, 2021
It would be easy to forget that back in 2019, BioNTech was an early-stage biotech firmly focused on cancer vaccines, before being catapulted onto the world-stage with its COVID-19 shot. The post BioNTech takes its first cancer vaccine into phase 2 appeared first on.

STAT
JUNE 28, 2023
The Gates Foundation unveiled plans Wednesday to fund a long-awaited trial for what, if proven effective, would be the first new tuberculosis vaccine in over a century. The 26,000-person, Phase 3 study, set to begin next year, will test a vaccine known as M72/AS01 that showed promising results from a smaller trial in 2019.

European Pharmaceutical Review
JANUARY 26, 2024
A paper, published in Nature Chemical Biology has reported the first ‘free-from ‘tree’ production of a key vaccine ingredient sourced from the soapbark tree. Bioengineering vaccine adjuvants The paper explained: “QS-21 is a potent vaccine adjuvant currently sourced by extraction from the Chilean soapbark tree.

European Pharmaceutical Review
AUGUST 2, 2023
A new COVID-19 vaccine has been authorised by the Medicines and Healthcare products Regulatory Agency (MHRA). Bimervax is now the ninth vaccine to be authorised by the UK’s independent medicines regulator to treat the virus. The vaccine demonstrated a strong immune response in the trial.

European Pharmaceutical Review
NOVEMBER 18, 2022
The World Health Organization (WHO)’s Global Vaccine Market Report 2022 , the first report to examine the impact of COVID-19 on the global vaccine market, shows that inequitable distribution is not unique to COVID-19 vaccines, with poorer countries consistently fighting to access vaccines in demand by wealthier countries.
Digital Pharmacist
AUGUST 16, 2022
Food and Drug Administration issued an emergency use authorization (EUA) for the JYNNEOS vaccine. Here is what you need to know about the monkeypox vaccine emergency use authorization. . JYNNEOS Vaccine Authorization Details . In this under 18 years old group, the JYNNEOS vaccine is administered subcutaneously.

pharmaphorum
JULY 21, 2022
GSK’s former head of vaccines R&D – Dr Emmanuel Hanon – is heading up a new biotech called Vicebio that will go up against his former employer with a vaccine against respiratory syncytial virus (RSV) infections. ” The post Ex-GSK vaccine chief to lead new UK biotech Vicebio appeared first on. . ” He said.

pharmaphorum
NOVEMBER 29, 2021
The UK’s Vaccines Manufacturing and Innovation Centre (VMIC) – unveiled with fanfare by the government in 2018 – is rumoured to be up for sale. It was billed at the time as a key tool to accelerate the development of new and innovative vaccines to combat some of the world’s most prevalent diseases, from discovery to licensed product.
pharmaphorum
DECEMBER 5, 2022
Valneva has moved a step closer to its goal of becoming the first company to file for approval of a vaccine against chikungunya virus in the US after reporting new clinical data today. Valneva estimates that the global market for vaccines against chikungunya could exceed $500 million annually by 2032.

European Pharmaceutical Review
AUGUST 24, 2023
GSK’s Shingrix (Recombinant Zoster Vaccine or RZV) vaccine has been shown to offer 100 percent efficacy against shingles in adults 50 years old and over in China. The Phase IV trial (ZOSTER-076) is the first-ever efficacy trial of the shingles vaccine Shingrix in China. Shingrix is a non-live, recombinant subunit vaccine.

European Pharmaceutical Review
SEPTEMBER 28, 2023
There has been a steady stream of developments in the vaccine manufacturing space throughout 2023. A notable milestone was Moderna’s announcement in April that it had, following finalisation of a ten-year strategic partnership with the UK Government, commenced construction of its mRNA vaccine manufacturing technology centre.
Pharmaceutical Technology
SEPTEMBER 15, 2022
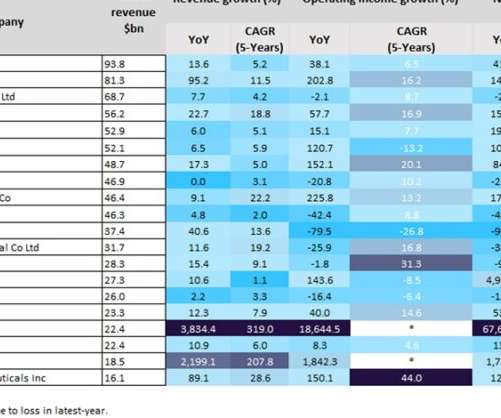
Last year was a positive year for biopharmaceutical companies, particularly those with Covid-19 vaccines. As a result of huge global sales of mRNA Covid-19 vaccines, the split in profits between Pfizer and BioNTech’s Comirnaty contributed towards revenues of $81.3bn and $22.4bn last year, respectively. YoY revenue growth.

Pharmaceutical Technology
FEBRUARY 21, 2023
Pfizer and BioNTech’s Covid-19 vaccine, Comirnaty, had a phenomenal year with forecast sales of $37bn in 2022. Comirnaty is the leading prophylactic vaccine for Covid-19 and is expected to generate an additional $2.8bn in sales in 2022 compared to GlobalData’s H1 2022 forecast. Comirnaty is the first globally approved Covid-19 vaccine.

Pharmaceutical Technology
JANUARY 5, 2025
Coronavirus Disease 2019 (COVID-19) vaccine is under clinical development by China National Biotec Group and currently in Phase III for Coronavirus Disease 2019 (COVID-19) Pneumonia.

STAT
JULY 3, 2024
Moderna scored a partial victory in its attempt to claim a share of billions of dollars in profits made by Pfizer and BioNTech from selling Covid-19 vaccine, after a judge in London ruled that one of its two patents was valid , according to Bloomberg News. Pfizer and BioNTech had filed a lawsuit in the U.K.

pharmaphorum
MARCH 11, 2022
After a period where vaccine development had fallen away, suddenly it is back in the limelight. However, for those individuals working on vaccine development, the pandemic has proven to be a validation of the important work being carried out in the field. billion from its vaccine in 2021. The ‘poor relation’.

Pharmafile
SEPTEMBER 28, 2023
The University of Birmingham hosted Bacterial Vaccines Network has been awarded £1.4m in funding from the UK government to accelerate the development of bacterial vaccines in the fight against antimicrobial resistance (AMR). This funding follows the Global AMR Innovation Fund’s (GAMRIF) £1.4m
Pharmaceutical Technology
JULY 15, 2024
Global vaccine coverage is yet to return to levels seen in 2019, shortly before the Covid-19 pandemic caused immunisation programme disruption.

pharmaphorum
JUNE 6, 2022
With vaccine hesitancy leading to a rise in measles cases, the FDA’s approval of GlaxoSmithKline’s venerable vaccine Priorix for sale in the US looks timely. The post FDA clears GSK’s Priorix, first new MMR vaccine in 50 years appeared first on. Photo credit: CDC/Dr Heinz F Eichenwald via Wikipedia.

Pharma Mirror
SEPTEMBER 22, 2021
Nantes, France, Naobios, a CDMO (Contract Development and Manufacturing Organization) providing bioprocess development and GMP production of clinical batches of viral vaccines BSL2/BSL3, oncolytic viruses and viral vectors, today announces the next phase in its partnership with FluGen, Inc., The company’s.

Pharmaceutical Technology
JANUARY 25, 2023
It was an exceptional year for Moderna’s vaccine Spikevax, with forecast sales of $19.5bn in 2022. Spikevax is an mRNA vaccine that is approved in 20 geographies including the US, EU, and, the UK. The vaccine first gained Emergency Use Approval in December 2020 in response to the Covid-19 pandemic.
Hospital Pharmacy Europe
OCTOBER 2, 2024
Pharmacist Dr João Gonçalves PhD considers the real-world evidence of a recent study demonstrating the feasibility and efficacy of a personalised peptide vaccine for glioblastoma – one of the most malignant primary brain tumours in adults.

pharmaphorum
MARCH 3, 2022
Pfizer has been awarded breakthrough status from the FDA for its respiratory syncytial virus (RSV) vaccine in pregnant women, putting the company in pole position to bring a shot to market that will protect infants from the life-threatening infection. The post After GSK stumble, Pfizer moves forward with RSV vaccine appeared first on.

Hospital Pharmacy Europe
DECEMBER 18, 2022
Vaccination against COVID-19 appears to provide a small but significant protection against the development of post-COVID-19 condition. Nevertheless, real-world evidence suggests that some vaccines, such as BNT162b2, have an effectiveness comparable to that reported in phase III clinical trials.

Pharmaceutical Technology
JANUARY 30, 2023
According to GlobalData’s Drugs database, there were 22 prophylactic vaccines in development for COVID-19 with sales forecasts available in H2 2022. Of these 22 vaccines, mRNA-derived vaccines dominate, with Comirnaty and Moderna’s Spikvax accounting for 88% of 2022 sales, with 58% and 30%, respectively.

PharmaShots
FEBRUARY 19, 2023
The NMPA approved it in Jan 2022 for individuals aged ≥3yrs.

Roots Analysis
OCTOBER 30, 2023
The next generation RNA therapeutics and RNA vaccines pipeline currently features the presence of more than 20 circular RNA therapeutics and vaccines, being developed across initial stages of drug development (discovery and preclinical).

Roots Analysis
NOVEMBER 6, 2023
In this context, several industry players have demonstrated high inclination towards supporting and advancing the development of next generation RNA-based therapeutics, vaccine and technologies. The below given figure provides an illustrative summary of the benefits of next generation RNA-based vaccines and RNA-based therapeutics market.

European Pharmaceutical Review
NOVEMBER 1, 2023
In July 2022, the World Health Organization (WHO) released its inaugural report on the pipeline of vaccines currently in development to prevent infections caused by antimicrobial resistance (AMR) bacterial pathogens. WHO referred to 61 bacterial vaccine candidates in diverse stages of clinical development. 1 WHO’s analysis was stark.

pharmaphorum
SEPTEMBER 22, 2022
Alexandre Le Vert, CEO and co-founder of Osivax, discusses the company’s breakthrough vaccine technology, oligoDOM, and how it’s driving the development of new influenza and SARS-CoV-2 vaccines that attack T-cells, providing a long-lasting effect. The technology. Antibodies cover the pathogen of the virus and neutralise it.

European Pharmaceutical Review
DECEMBER 19, 2023
Many of today’s vaccines are produced in ready-to-inject liquid formulations that must be kept cold to maintain stability. However, this greatly complicates both the worldwide distribution and stockpiling of vaccines and other drugs. A requisite cold chain has been designed and implemented to be uninterrupted from factory to patient.

pharmaphorum
DECEMBER 6, 2022
5-adapted bivalent COVID-19 vaccine for children 6 months through 4 years of age to the FDA as a third 3-µg-dose in a three-dose-primary series, following two 3-µg-doses of the original vaccine. It is thought the updated vaccine might help to prevent severe illnesses and hospitalisation. 5-adapted bivalent vaccine.

Hospital Pharmacy Europe
JANUARY 26, 2023
Despite HIV having been first discovered in 1983 , there are still no vaccines available against the virus. The vaccine used a common-cold virus (adenovirus serotype 26, Ad26) and was designed to deliver four antigens to illicit an immune response. The aim of the study was to evaluate the efficacy of a heterologous vaccine, Ad26.Mos4.HIV,

pharmaphorum
JULY 27, 2021
Flushed with the success of its COVID-19 vaccine, BioNTech has pressed the accelerator on the development of shots for other infectious diseases, and now plans to take malaria and tuberculosis candidates into the clinic next year. The post BioNTech’s mRNA vaccine drive now includes malaria, TB shots appeared first on.
European Pharmaceutical Review
AUGUST 15, 2022
According to Reuters , Kenya, Tanzania, Senegal and Cameroon have either run out of or are close to running out of vaccines to protect children against the deadly rotavirus infection following undisclosed “manufacturing challenges” at GSK. We are witnessing the largest sustained drop in childhood immunisation in a generation.

Pharmaceutical Technology
MAY 25, 2023
A new vaccine developed by the Serum Institute of India to fight meningococcal disease could help eliminate meningitis across Africa. The results from a trial, published in The New England Journal of Medicine , found the vaccine was associated with a strong immune response and good safety profile. percentage points (96% CI, −0.3

pharmaphorum
MAY 30, 2022
Shares in Swedish biotech RhoVac were down 94% in mid-morning trading today after the company revealed a phase 2b trial of its prostate cancer vaccine RV001 showed it was no more effective than placebo. The post RhoVac pummelled as lead cancer vaccine fails study appeared first on.

BuzzRx
SEPTEMBER 12, 2022
There are two types of meningococcal vaccines available in the United States. Please keep reading to learn more about these vaccines, including their uses, benefits, and side effects. Meningococcal disease is relatively rare in the US, with 375 cases reported in 2019. What are the different types of meningitis vaccines?

STAT
OCTOBER 27, 2022
The investigation began in February and covers 2017, 2018, and 2019. The Italian branch allegedly sent the capital to foreign affiliates linked to a units based in Delaware called Pfizer Production and Pfizer Manufacturing. Continue to STAT+ to read the full story…
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content