Dengue Vaccine: What Pharmacists Need to Know
Pharmacy Times
JUNE 27, 2022
The FDA approved the first vaccine for dengue disease in May 2019.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmacy Times
JUNE 27, 2022
The FDA approved the first vaccine for dengue disease in May 2019.

STAT
JULY 3, 2024
But while Alzheimer’s medicines are making it through the FDA, they face logistical hurdles that can slow their use and in some cases, skepticism about the balance between their benefits and risks. seeking to invalidate two patents behind Moderna’s vaccine, Spikevax, claiming they were not novel.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
DECEMBER 5, 2022
Valneva has moved a step closer to its goal of becoming the first company to file for approval of a vaccine against chikungunya virus in the US after reporting new clinical data today. Valneva estimates that the global market for vaccines against chikungunya could exceed $500 million annually by 2032.
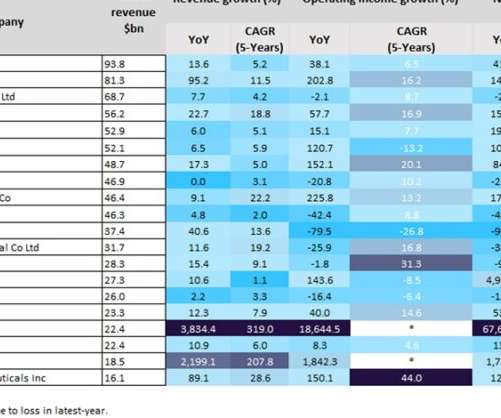
Pharmaceutical Technology
SEPTEMBER 15, 2022
Last year was a positive year for biopharmaceutical companies, particularly those with Covid-19 vaccines. As a result of huge global sales of mRNA Covid-19 vaccines, the split in profits between Pfizer and BioNTech’s Comirnaty contributed towards revenues of $81.3bn and $22.4bn last year, respectively. YoY revenue growth.

pharmaphorum
JUNE 6, 2022
With vaccine hesitancy leading to a rise in measles cases, the FDA’s approval of GlaxoSmithKline’s venerable vaccine Priorix for sale in the US looks timely. Most people get better after contracting it, but in a small proportion of patients it can lead to life-threatening conditions like pneumonia and meningitis.

pharmaphorum
MARCH 3, 2022
Pfizer has been awarded breakthrough status from the FDA for its respiratory syncytial virus (RSV) vaccine in pregnant women, putting the company in pole position to bring a shot to market that will protect infants from the life-threatening infection. AZ, Sanofi antibody aces phase 3 trial. Last year, sales were around $273 million.

pharmaphorum
DECEMBER 6, 2022
Pfizer and BioNTech have announced that they have submitted an Emergency Use Authorisation (EUA) to the US Food and Drug Administration (FDA) for their Omicron BA.4/BA.5-adapted It is thought the updated vaccine might help to prevent severe illnesses and hospitalisation. 5-adapted bivalent vaccine. 5-adapted bivalent vaccine.

STAT
OCTOBER 27, 2022
The investigation began in February and covers 2017, 2018, and 2019. The Italian branch allegedly sent the capital to foreign affiliates linked to a units based in Delaware called Pfizer Production and Pfizer Manufacturing. Continue to STAT+ to read the full story…

pharmaphorum
MAY 13, 2022
Way back in January 2020, Pfizer’s 2019 annual report could only note the potential disruption from a novel strain of coronavirus. In March 2020 Pfizer began collaborating with BioNTech on a vaccine for COVID-19 and 269 days later it received emergency use authorisation from the FDA.

STAT
NOVEMBER 18, 2022
The document says that public funding for the development of vaccines and treatments should be more transparent, and include provisions to ensure that any resulting products are distributed evenly around the world. As of 2019, about 1.9 The first therapy that delays the onset of type 1 diabetes received approval from the U.S.

PharmaShots
MAY 23, 2023
Stock Exchange: TYO With focus areas extending to the discovery and development of biopharma products, AnGes is dedicated to developing genetic medicines and therapeutic vaccines for intractable or rare diseases. The company received its first approval from the Japanese government in 2019 to treat critical limb ischemia.

pharmaphorum
FEBRUARY 19, 2021
A global emergency stockpile of Ebola vaccine is being deployed to curb the epidemic, although experts are confident that synergies in public health practises will ensure a rapid response to both. The injectable single-dose Ebola vaccine is manufactured by MSD, known as Merck & Co in the US and Canada.

Pharmaceutical Technology
SEPTEMBER 5, 2022
Following AstraZeneca’s success in vaccine development during the Covid-19 pandemic , the pharmaceutical giant is now looking to expand its scope through acquisitions across a range of indications, says CEO Pascal Soriot. “We The FDA has granted the drug an orphan drug designation, and Phase III trials with eplontersen were completed In June.

pharmaphorum
FEBRUARY 23, 2022
The global health crisis caused spending on healthcare to increase , due to the funding provided to the development and purchases of vaccines and treatments, as well as support provided for the associated infrastructure. In 2020, AMA’s research showed that savings from the use of biosimilars tripled from 2019.

pharmaphorum
AUGUST 8, 2022
Oxbryta came to market in 2019, a few days after Novartis’ injectable anti-P-selectin antibody Adakveo (crizanlizumab), which is also tipped for blockbuster sales but like Oxbryta has suffered from a slow rollout. It comes shortly after the group closed a $6.7

pharmaphorum
SEPTEMBER 8, 2020
Governments do not have to look hard to find ample opportunity for their health systems to accelerate biosimilar adoption practices and drive huge healthcare savings while still delivering excellent high quality patient care, as noted openly by the FDA during the last 18 months. Published 8 March 2019. Published 25 November 2019.
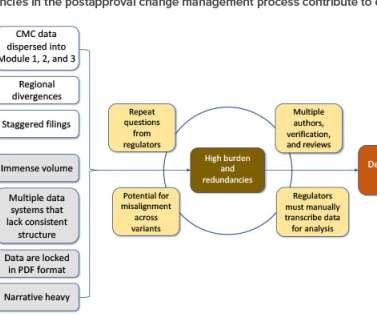
ISPE
AUGUST 29, 2022
In an attempt to address these challenges, in 2019 the ICH endorsed ICH Q12, Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (and Annexes). In May 2021, the US Food and Drug Administration (FDA) published a draft industry guidance, ICH Q12: Implementation Considerations for FDA-Regulated Products.

pharmaphorum
APRIL 16, 2021
Even with the vaccine roll-out, further waves of COVID infection and lockdowns mean these vital drivers of launch performance remain impacted in 2021, and even beyond. Approval by regulators such as the FDA or EMA, as reported by the regulators themselves. We measured the pandemic impact on the launch of NAS at four levels.

pharmaphorum
DECEMBER 22, 2021
Japanese drugmaker Takeda has suffered a blow to its late-stage pipeline, after the FDA rejected its marketing application for TAK-721, a drug candidate that is trying to be the first approved therapy in the US for eosinophilic oesophagitis (EoE). Takeda acquired the drug as part of its $62 billion merger with Shire in 2019.

European Pharmaceutical Review
JANUARY 29, 2024
During August 2023 the FDA also updated its guidance for NDSRIs 7 and it was closely aligned (but not identical) to EMA’s evolving guidance. 3 Differences include that potency category 1 in FDA’s CPCA approach is 26.5 FDA has also published the AIs of some 265 NDSRIs. ng/day, compared to EMA which is 18 ng/day.

European Pharmaceutical Review
JULY 17, 2023
The $82 million project is funded by the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research. This $85 million programme ran between 2007 and 2019. This new pilot-scale system builds on success of the Novartis-MIT Center for Continuous Manufacturing.

pharmaphorum
MARCH 9, 2021
Regeneron and Eli Lilly have already got antibody therapies on the market following emergency approvals by regulators including the FDA but pharma companies are beginning to focus on the threat posed by emerging variants. Ablynx, which is now a subsidiary of Sanofi following a $4.8

PharmaShots
MARCH 14, 2023
4 Government Incentive Programs FDA (USA): The orphan drug act was passed in January 1983, with a joint effort of the National Organization for Rare Disorders (NORD), and other organizations. The act includes drugs, vaccines, and therapeutics that are intended to treat a disease affecting <200,000 American citizens.

ISPE
JUNE 6, 2023
Our speakers will give insights on hospital manufacturing and commercial manufacturing of ATMPs/C>s, including networking opportunities and a panel discussion with all speakers from the track featuring Peter Marks from the FDA as a special guest speaker.

PharmaShots
FEBRUARY 21, 2023
In Aug’21, the US FDA cleared QSam’s IND application for CycloSam. In Jun’21, the company initiated the evaluation of FAP-2286 in a P-I/II clinical trial (LuMIERE) following the US FDA’s IND clearance. In Sep’21, the US FDA granted DUNP19 an ODD for Osteosarcoma.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. Brand drugs, generic drugs, and medical devices alike have all been the target of state failure-to-warn litigation; in a recent case, OTC acetaminophen is the target. But we digress.

pharmaphorum
JANUARY 27, 2022
Belgium-born Stoffels will join Galapagos after a challenging period for the company, which is in the throes of launching its JAK1 inhibitor Jyseleca (filgotinib) for rheumatoid arthritis in Europe and Japan, but not the US after the FDA rejected the drug amid safety concerns.
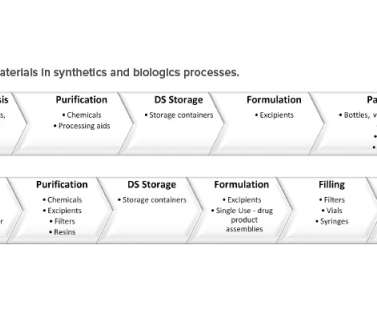
ISPE
AUGUST 30, 2022
Recent US FDA data show that the lack of raw material availability contributes to 27% of drug shortages (see Appendix ). It is considered a moderate change by the FDA (CBE30) and NMPA. This change ranges from a moderate change (CBE-30) by the FDA to a minor change not requiring prior approval by the WHO.

PharmaShots
MARCH 9, 2023
She has also been awarded Crain’s Notable Women in Healthcare in 2019. Since its launch in 2016, Tivic Health has obtained three regulatory clearances for ClearUP, including FDA 510(k), FDA De Novo, and CE Mark, four patents, and numerous awards. LinkedIn Total experience: 31 yrs.
pharmaphorum
AUGUST 16, 2021
Nearly every element of the clinical trial design, data collection, analysis and approvals has been shortened and this helped ensure the swift rollout of safe and effective vaccines.”. In 2019, Formedix collaborated with a UK-based consortium interested in observing early safety data. About the interviewee.

Pharmaceutical Technology
DECEMBER 27, 2022
Once lockdown measures eased and Covid-19 vaccines and boosters became available, the industry anticipated a significant recovery in the market and in the number of deals. In this deal, BMS gained Turning Point’s lead asset, repotrectinib, which has already received three FDA Fast Track designations.
The Checkup by Singlecare
OCTOBER 9, 2024
Food and Drug Administration (FDA) in 2019 and is made by the pharmaceutical company Janssen Pharmaceuticals. Spravato is also used to treat symptoms of depression in adults with depression who are having suicidal thoughts or behaviors. Spravato is a brand-name drug, and there is no generic available. Spravato was approved by the U.S.

pharmaphorum
AUGUST 8, 2022
If confirmed, it will be another example of Pfizer leveraging the windfall cash generated by its COVID-19 vaccine Comirnaty and oral antiviral therapy Paxlovid to beef up its pipeline of new therapies, coming a few months after it closed a $6.7 billion acquisition of Arena Pharma and made an $11.6 billion takeover bid for Biohaven.

ISPE
OCTOBER 26, 2022
ATMP facilities are different from conventional pharmaceutical facilities that process other traditional modalities, such as vaccines and monoclonal antibodies (mAbs), and often require heightened segregation and smaller footprints. FDA CFR Title 21 Parts 211, 600, and 1271; 8. , Complete Data Gathering.

ISPE
SEPTEMBER 11, 2023
2 October 2019. Manufacturing Biological Medicines on Demand: Safety and Efficacy of Granulocyte Colony-Stimulating Factor in a Mouse Model of Total Body Irradiation.” “Sanofi Pumps $554M into New Vaccine Manufacturing Facility in Eastern France.” New Project: Evolutive Vaccines Facility for Sanofi.” BioPharm International.
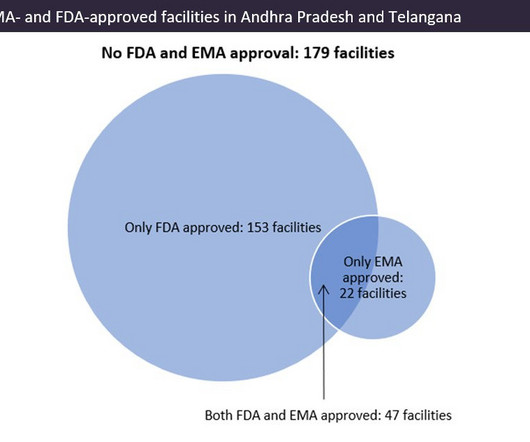
Pharmaceutical Technology
APRIL 4, 2023
Manufacturing growth in the region Since our last analysis in 2019 (‘Andhra Pradesh and Telangana: Indian Contract Manufacturing Powerhouses for US API Supply’, EMOR, September 2019), the two states have opened an extra 49 manufacturing sites for foreign markets. © GlobalData. ©GlobalData.

pharmaphorum
JANUARY 31, 2022
Pfizer paid $250 million upfront for rights to vupanorsen in 2019, in a deal that had another $1.3 There were however also some worrying safety signals, including increases in liver fat and enzymes, that seemed to be dose-dependant, linking them to drug treatment, which were referenced by Pfizer in its statement on the decision.

The FDA Law Blog
SEPTEMBER 30, 2024
Another rejected proposal was a definition of “vaccine” (vaccines are exempt from Medicaid rebates), which would have limited this term to a product that is administered prophylactically – i.e., to prevent rather than treat a disease. The most noteworthy are the following: 1. See 42 U.S.C. 1396r-8(b)(3)(C)(iii).

Pharmaceutical Technology
MAY 15, 2023
million global hospitalisations in 2019. Nirsevimab is still undergoing regulatory review in the US, with the US Food and Drug Administration (FDA) accepting the Biologics License Application (BLA) for nirsevimab in January 2023. RSV is the most common cause of LRTD. Other companies are competing in the RSV therapy space, too.

Pharmaceutical Technology
MARCH 2, 2023
is under development also treatment of pneumonia,coronavirus disease (2019). It is administered through subcutaneous route.It It is under development for the treatment of human papillomavirus (HPV)-associated recurrent respiratory papillomatosis (RRP).

PharmaShots
APRIL 13, 2023
Its subsidiary, DePuy's CHARITE Artificial Disc for degenerative disc disease was the first device to be approved by the US FDA By acquiring Roche’s OTC business, Bayer was able to strengthen its consumer health business. Its top 8 products contributed more than $1B to the company’s net revenue. compared to 2015.

PharmaShots
JANUARY 31, 2023
MyoVista wavECG is the company’s first FDA-cleared product which is designed to provide diagnostic information related to cardiac dysfunction in addition to being a resting 12-lead ECG. LP-310, another lead asset of the company received FDA Type-B Pre-IND guidance recently for Oral Lichen Planus. Founded Year: 2019 No.
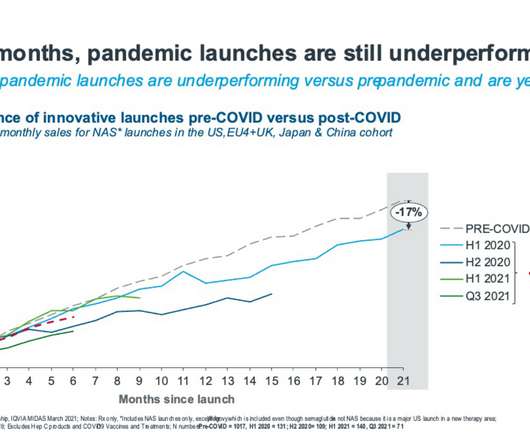
pharmaphorum
JUNE 29, 2022
COVID vaccines and treatments have created a substantial market over and above the existing Rx market- IQVIA estimates that the cumulative value of COVID vaccines could be between $185 and 295bn to 2026. The prescription medicine market has recovered from the wild swings of the early pandemic with renewed growth.

Pharmaceutical Technology
MARCH 22, 2023
According to the GlobalData report Contract Injectable Packaging Trends in the Bio/Pharma Industry , more than half (55%) of FDA drug approvals in 2021 were accounted for by injectables. This was higher than the number of approvals in 2019 and 2020. The injectable drugs market is currently valued at around $532 billion.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content