FDA-Approved Labeling: Is Enough Enough?
The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

pharmaphorum
JULY 20, 2021
The US regulator had extended its review of the drug by three months – setting back its action date from April to 29 July – but now says that deficiencies in the marketing application “preclude discussion” of labelling and post-marketing requirements. “Despite….advantageous
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
OCTOBER 10, 2022
Javitt — On September 28, 2022, the FDA issued the long anticipated final Clinical Decision Support Software Guidance (CDS Guidance), which replaces the revised draft guidance document from 2019. Medical information about a patient. Other medical information.

Pharmaceutical Technology
JULY 29, 2022
Dr Jeremy Veenstra-VanderWeele, professor of developmental neuropsychiatry at the Columbia University Irving Medical Center, explains that agitation is seen in the minority of autistic teens, who struggle with communication. An inability to clearly communicate with others and express their wants and needs results in frustration, he adds.

The Checkup by Singlecare
DECEMBER 15, 2022
Google Survey , 2019). The Cyberbullying Research Center , 2019). The Cyberbullying Research Center , 2019). The Cyberbullying Research Center , 2019). UNICEF Poll , 2019). high school students experienced cyberbullying in 2019. In another survey in 2019, 15.7% Ditch the Label , 2017).

pharmaphorum
FEBRUARY 5, 2021
The study was carried out at the request of the FDA, after concerns emerged about the safety of Xeljanz which emerged in 2019, and specifically a possible increased risk of blood clots in the lungs and death, particularly with the higher 10mg approved dose of the drug.

The Checkup by Singlecare
NOVEMBER 30, 2023
Available under the brand names Neurontin , Gralise , and Horizant , gabapentin is also commonly prescribed off-label for general nerve pain, migraines , and bipolar disorder. Off-label use is when a drug is used for a purpose other than what it is approved for. Side effects of CNS depressants include drowsiness and trouble breathing.

pharmaphorum
SEPTEMBER 6, 2022
billion in 2019. The first principle, labelled the “Science of the Human”, asserts that self-care products should be rooted in a thorough understanding of human biology and medical insights, while the second, the “Science of Regulation”, calls for independent regulation.

The Checkup by Singlecare
JUNE 23, 2023
How gabapentin works Gabapentin works by acting on neurotransmitters—chemicals produced by the nerve cells in your brain—and changing how those nerve cells communicate with each other. It’s essential to read the label before you pop that pill (or oral solution) and take it as advised. When in doubt, ask your pharmacist.

The Checkup by Singlecare
JANUARY 23, 2024
Communicate these lists to every healthcare provider that cares for you. When to talk to a healthcare provider about amlodipine interactions Besides keeping and communicating lists, you should ask about interactions every time you are prescribed a new medication or are about to take an over-the-counter product.

The Checkup by Singlecare
DECEMBER 12, 2023
The Food and Drug Administration (FDA) even sent a special communication to call attention to this risk. Taking an active role in monitoring interactions and communicating concerns can minimize your risk. For that reason, it is typically best to avoid combining opiates with zolpidem.

pharmaphorum
MARCH 15, 2022
The COVID-19 pandemic has radically disrupted traditional medical society conferences in a way that could scarcely have been imagined in 2019. The majority of societies reported significant increases in attendee numbers compared with those from their equivalent 2019 physical conferences. The ‘hybrid’ label is confusing.
European Pharmaceutical Review
OCTOBER 5, 2022
And beyond the physical limitations, those instigating industry-wide change also face pushback in the boardroom, with a recent study citing poor communication between stakeholders and rigid practices among the key barriers to more circular product packaging. 2019 [cited 2022Sep]. Consultancy.uk; 2021 [cited 2022Sep]. Overstreet K.

The Checkup by Singlecare
JANUARY 18, 2024
Hence, it is important to communicate openly with your healthcare providers about all medications, vitamins, and supplements you take to ensure proper medical advice and monitoring. Eliquis and grapefruit Grapefruit and grapefruit juice interact with the effectiveness of many medicines due to binding to the enzyme CYP3A4.

The FDA Law Blog
JULY 10, 2023
FDA communicates via a Substantive Interaction to inform the submitter either that FDA will proceed with Interactive Review or that the 510(k) will be placed on hold until FDA receives a complete response to an Additional Information request. For labeling documents and the 510(k) summary, FDA often requests both clean and redlined versions.
European Pharmaceutical Review
SEPTEMBER 22, 2023
Since 2019, she has been Head of Vaccines and Immunoanalytical Sciences at AC Immune. Yves joined AC Immune in 2020 and is now Manager, Investor Relations and Communications. Marie Kosco-Vilbois, PhD Marie Kosco-Vilbois joined AC Immune as Chief Scientific Officer in 2019. Brain Communications.2022;4(1). Wang CY, et al.

The Checkup by Singlecare
AUGUST 4, 2023
Anticholinergic drugs “Acetylcholine is a chemical messenger that allows our nerve cells to communicate with one another and carry out essential functions within our bodies,” says Whitney Prude, Pharm.D. Double check labels, or better yet, ask your pharmacist to make sure you aren’t doubling up on diphenhydramine.

pharmaphorum
OCTOBER 4, 2022
The final guidance follows the draft CDS software guidance issued on the same date in 2019. Software functions intended to display, analyse, or print medical information about a patient or other medical information (e.g. Additionally, relevant sources should be identified and made available to the HCP user.

pharmaphorum
FEBRUARY 26, 2021
Robert Wood’s granddaughter, Mary Lea, was the first baby to be used on the baby powder label. billion) hit the company in 2019. Johnson’s Baby Powder also went on sale during this year and was extremely successful. Litigation & controversy.
Hospital Pharmacy Europe
FEBRUARY 22, 2024
Their training and skills enable them to interpret and communicate complex medicines-related information to patients and other health professionals. Gene 2019 Nov 15;718:144050 Clinical Pharmacogenetics Implementation Consortium. Pharmacogenomics: Relevance and opportunities for clinical pharmacology. Introduction to pharmacogenetics.
The Checkup by Singlecare
OCTOBER 30, 2023
In addition, sertraline is prescribed for several other off-label indications, including binge eating disorder and body dysmorphic disorder, even sexual dysfunction conditions like premature ejaculation. Therefore, any onset of symptoms should be managed by immediate communication with a healthcare professional.
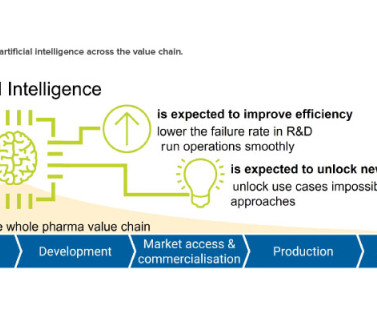
ISPE
JULY 10, 2023
In sections where we applied ChatGPT for example cases, we indicate this as such: The question or remark from us is labeled as Authors. This so-called zero-shot approach has the strong advantage that no data must be labeled for the tailoring step and that the same model can be used for many different NLP applications.

The Thyroid Pharmacist
OCTOBER 28, 2022
Dose: While the label of the product recommends taking two capsules per day, I used higher doses, building up to four capsules, three times per day. The probiotic has been so successful in inducing remission, it has been labeled as a “medical food.” doi:10.1136/gutjnl-2019-318427. doi:10.1136/gutjnl-2019-318427.
ISPE
OCTOBER 27, 2022
Wireless medical devices use wireless radio frequency communication such as wifi, Bluetooth, and cellular/mobile phone technology. However, for certain DHTs classified as devices or SaMD, ISO 14971:2019 specifies the terminology, principles, and process for application of risk management. Is the software MDSW according to MDCG 2019-11?
ISPE
OCTOBER 27, 2022
Wireless medical devices use wireless radio frequency communication such as wifi, Bluetooth, and cellular/mobile phone technology. However, for certain DHTs classified as devices or SaMD, ISO 14971:2019 specifies the terminology, principles, and process for application of risk management. Is the software MDSW according to MDCG 2019-11?

The Checkup by Singlecare
JANUARY 23, 2024
Although it’s not officially approved for female use, some healthcare providers may prescribe it off-label for female sexual dysfunction. The FDA label for Viagra explicitly states it’s not indicated for women or children. Communicating with a partner may also help alleviate anxiety and improve intimacy. Can women take Viagra?

ISPE
MARCH 29, 2023
Published November 2019. Challenges with the Current Module 2 Structure The US FDA published a white paper in 2018 calling for a revision to Module 2 because, “there can be a disconnect between applicants and regulators regarding the communication of quality data and its impact on the assessment.
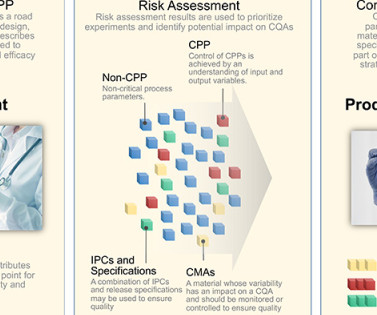
ISPE
MARCH 29, 2023
Published November 2019. Challenges with the Current Module 2 Structure The US FDA published a white paper in 2018 calling for a revision to Module 2 because, “there can be a disconnect between applicants and regulators regarding the communication of quality data and its impact on the assessment.
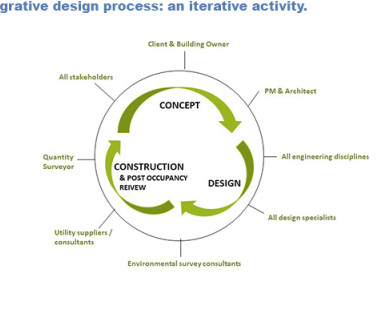
ISPE
MARCH 15, 2023
query=environment Labeling and Organization There are various ways to group the many types of environmental sustainability challenges and goals. Table 2: Organization and labeling examples for environmental sustainability issues. 24 September 2019. Wiley (2019). New Biotechnology 49 (2019): 37–42. 1 July 2019.

ISPE
MAY 10, 2023
1 May 2023 Funded by the European Commission from 2019, the Smart Pharmaceutical Manufacturing Project (SPuMoNI) 1 harnesses the potential of state-of-the-art technologies for the pharmaceutical industry. 3M explores New Label-As-A-Service Concept with Blockchain on Azure to Stop Counterfeit Pharmaceuticals.” Chis, Ph.D. Microsoft.com.

The Thyroid Pharmacist
OCTOBER 6, 2023
Thus, this article was due for an update, as it was initially published in 2019. Many readers in my community report symptoms of histamine intolerance, which can be challenging to differentiate from other food sensitivities and imbalances in the digestive system. Published 2019 Mar 29. 2019; 73(1): 102-104. March 2019.

The Thyroid Pharmacist
JANUARY 6, 2023
It breaks my heart to think about the many thyroid patients who get labeled as clinically depressed instead of being tested for thyroid antibodies, and to think of the patients who start on thyroid hormones without consideration of dosage and form, resulting in ongoing symptoms, including depression. 2019 Jun 19;:]. JAMA Psychiatry.

The Thyroid Pharmacist
OCTOBER 27, 2023
These signals create communication and function within nerves and muscles, as the electrolytes move in or out of cells. Precautions Do not take more than the recommended dose on the label. Humic extract, the main ingredient, contains fulvate, which helps the cells in our gut better communicate with each other and bacteria.

PharmaShots
APRIL 3, 2023
The system offers alternative options for those patients receiving other medications The system was originally approved in 2019 for treating chronic pain.
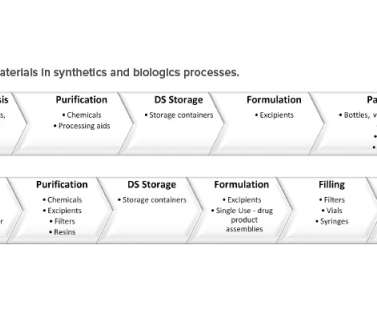
ISPE
AUGUST 30, 2022
Published November 2019. The original molecular weight (MW) specification for polypropylene glycol 2000 (PPG) of 1800–2200 was based on the Food Chemical Codex monograph (90%–110% of label) and not based on a scientific understanding of the process/product requirements. You may unsubscribe from these ISPE communications at any time.

The Thyroid Pharmacist
JANUARY 20, 2023
The first case is outlined in a 2019 report by Roberto Vita and his research team, and published in Revista da Associação Médica Brasileira. Markomanolaki and published in a 2019 issue of Journal of Molecular Biochemistry. Published 2019 Jul 22. Published 2019 Dec 9. Published 2019 Aug 15. vs. 398 U/mL). [1]

The Thyroid Pharmacist
FEBRUARY 10, 2023
Both our sex and thyroid hormones are part of an overall hormone communications network in our body, referred to as the HPA (Hypothalamic-Pituitary-Adrenal) axis. To date, vaginally-applied DHEA is still only available through prescription, either as Intrarosa® or an off-label customized prescription. Published 2019 Dec 17.

Pharmaceutical Technology
NOVEMBER 30, 2022
The Health Survey for England 2019 reported that 28% of adults in the UK were obese and 36.2% weight reduction in obese individuals, as per its label. Furthermore, in order to make up for shortages Novo Nordisk’s Ozempic, a version of semaglutide that is approved to treat type 2 diabetes, was used off-label for obese patients.

Pharmaceutical Technology
NOVEMBER 30, 2022
The Health Survey for England 2019 reported that 28% of adults in the UK were obese and 36.2% weight reduction in obese individuals, as per its label. Furthermore, in order to make up for shortages Novo Nordisk’s Ozempic, a version of semaglutide that is approved to treat type 2 diabetes, was used off-label for obese patients.

Pharmaceutical Technology
DECEMBER 22, 2022
However, he did point out that the joint assessment could be a useful way of narrowing down typically broad EMA label content to specific patient populations. He believed that EUnetHTA’s conclusions will most likely influence the pricing and reimbursement decision of the drug at the national level. Free Case Study.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content