First CAR T-cell therapy recommended on NHS
European Pharmaceutical Review
JANUARY 26, 2023
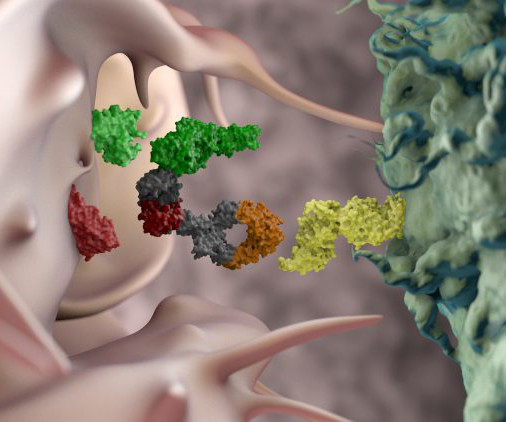
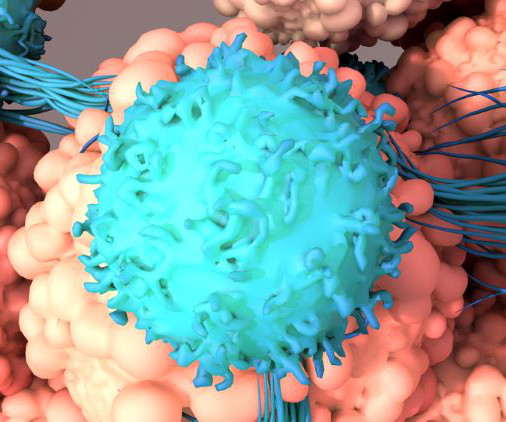
CAR T-cell therapy engineers a patient’s own immune cells (T-cells) to detect, target and destroy cancer cells. Of those who receive a transplant, about 50 percent will ultimately relapse, stated research from 2017, published in the journal Blood. Could CAR T therapies be manufactured in one day?






























Let's personalize your content