A Proposal for a Comprehensive Quality Overall Summary
ISPE
MARCH 29, 2023
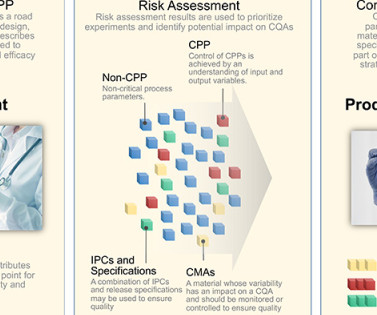
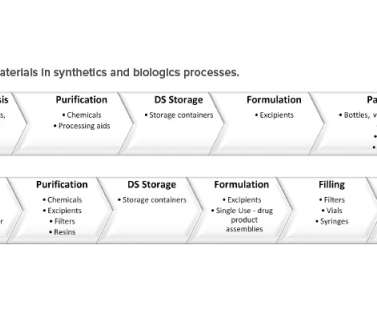
Application of this innovative approach will quickly orient regulators to the content of Module 3, “present product quality benefit-risk considerations, summarize the pharmaceutical development, present an overall understanding of the product quality,” 1 and facilitate continuous improvement. 2005N–0262) Federal Register (2005).









Let's personalize your content