FDA’s Proposal to Remove Oral Phenylephrine from the OTC Monograph Isn’t a Surprise but What is Left “Over-the-Counter”?
The FDA Law Blog
NOVEMBER 11, 2024
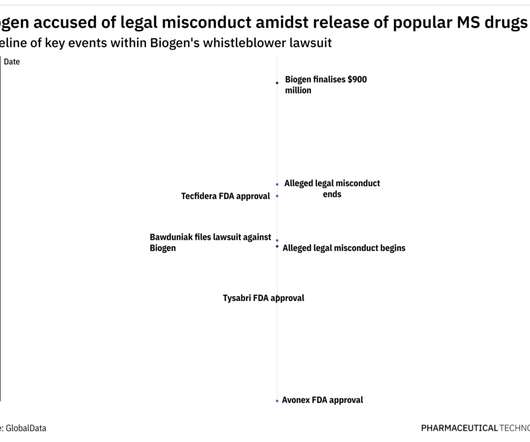
Livornese & JP Ellison — On November 8, 2024, FDA issued a proposed order to remove the oral decongestant ingredient phenylephrine (including both phenylephrine hydrochloride and phenylephrine bitartrate) (collectively, PE) from the OTC monograph on the basis of a lack of effectiveness. By Deborah L. a); OTC Monograph M012: § M012.20.






















Let's personalize your content