Contamination Trends & Proposed Solutions
ISPE
MARCH 8, 2023
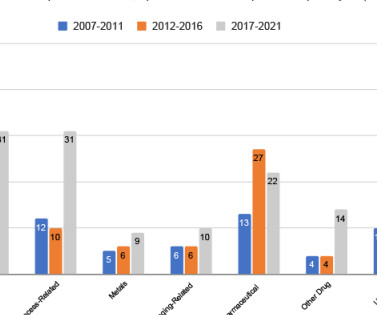
5 , 8 Compounding pharmacies were commonly mentioned as sources of microbial cross-contamination, especially in the US. 9 , 15 This has led to compounding pharmacies completing high-volume activities such as mass repackaging, thereby increasing the risk of cross-contamination. Compounding Pharmacies: Who Is in Charge?”















Let's personalize your content